Neutrophil-to-lymphocyte ratio-based prognostic score can predict outcomes in patients with advanced non-small cell lung cancer treated with immunotherapy plus chemotherapy
- PMID: 40234811
- PMCID: PMC11998248
- DOI: 10.1186/s12885-025-13811-y
Neutrophil-to-lymphocyte ratio-based prognostic score can predict outcomes in patients with advanced non-small cell lung cancer treated with immunotherapy plus chemotherapy
Abstract
Backgroud: Immune checkpoint inhibitor (ICI) plus chemotherapy has become the standard of care for advanced non-small cell lung cancer (NSCLC). Nonetheless, reliable efficacy biomarkers of ICI plus chemotherapy are lacking. In this research, we sought to explore efficacy biomarkers and construct robust prognostic models in NSCLC patients treated with ICI plus chemotherapy.
Methods: We retrospectively analyzed 171 patients with advanced NSCLC treated with ICI plus chemotherapy. Clinical characteristics and peripheral blood inflammatory indexes were collected and prognostic models were constructed to explore efficacy and prognosis biomarkers of ICI plus chemotherapy.
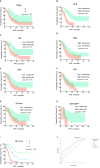
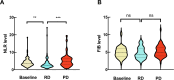
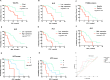
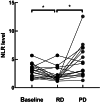
Results: In the cohort that received first-line ICI plus chemotherapy, pre-treatment neutrophil-to-lymphocyte ratio (NLR) > 3.3 and fibrinogen (FIB) > 3.196 were associated with worse efficacy and were independent risk factors of progression-free survival (PFS). Compared to programmed cell death ligand 1 (PD-L1), the derived NLR-FIB (NF) score had significantly improved accuracy in predicting efficacy and prognosis. In advanced NSCLC patients with targetable oncogenic driver alterations receiving second- or post-line ICI plus chemotherapy, pre-treatment NLR > 3.53 was associated with worse efficacy and was an independent risk factor of PFS and OS; Tyrosine kinase inhibitor (TKI)-PFS > 12 months were independent risk factors of overall survival (OS). Secondary epidermal growth factor receptor (EGFR)-T790M mutation, platelet-to-lymphocyte ratio (PLR) > 196.81 and albumin (ALB) < 40.25 were associated with worse PFS. Based on NLR and TKI-PFS, an NLR-TKI-PFS (NTP) score was constructed with three OS risk prognosis categories: favorable, intermediate, and poor (corresponding to a median OS of 21, 12, and 5.3 months).
Conclusions: The noninvasive NF score, combining NLR > 3.3 and FIB > 3.196, was superior to PD-L1 estimated from tumor tissue in predicting the efficacy and prognosis of first-line ICI plus chemotherapy in advanced NSCLC patients. The noninvasive NTP score, combining NLR > 3.53 and TKI-PFS > 12 months, is a valuable tool for predicting OS and PFS in advanced NSCLC patients with targetable oncogenic driver alterations receiving second- or post-line ICI combination therapy.
Keywords: Efficacy prediction; Immunochemotherapy; Neutrophil lymphocyte ratio; Non-small cell lung cancer; Prognostic biomarker.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the Ethics Committee of Nanfang Hospital (NFEC-BPE-098). This is an observational and retrospective study that has obtained exemption from informed consent from the Ethics Committee. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab.Lung Cancer. 2017 Sep;111:176-181. doi: 10.1016/j.lungcan.2017.07.024. Epub 2017 Jul 24. Lung Cancer. 2017. PMID: 28838390
-
Efficacy of ICI-based treatment in advanced NSCLC patients with PD-L1≥50% who developed EGFR-TKI resistance.Front Immunol. 2023 May 17;14:1161718. doi: 10.3389/fimmu.2023.1161718. eCollection 2023. Front Immunol. 2023. PMID: 37266427 Free PMC article.
-
Relationship between the combination of platelet count and neutrophil-lymphocyte ratio and prognosis of patients with advanced non-small cell lung cancer treated with immune checkpoint inhibitors plus chemotherapy: A retrospective cohort study.Thorac Cancer. 2024 Oct;15(28):2049-2060. doi: 10.1111/1759-7714.15437. Epub 2024 Aug 28. Thorac Cancer. 2024. PMID: 39193939 Free PMC article.
-
Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2020 Dec 14;12(12):CD013257. doi: 10.1002/14651858.CD013257.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 30;4:CD013257. doi: 10.1002/14651858.CD013257.pub3. PMID: 33316104 Free PMC article. Updated.
-
The landscape of immune checkpoint inhibitor plus chemotherapy versus immunotherapy for advanced non-small-cell lung cancer: A systematic review and meta-analysis.J Cell Physiol. 2020 May;235(5):4913-4927. doi: 10.1002/jcp.29371. Epub 2019 Nov 6. J Cell Physiol. 2020. PMID: 31693178 Free PMC article.
Cited by
-
Increased Pre-Operative Lung Immune Prognostic Index Score Is a Prognostic Factor in Cases of Pathological T3 Renal Cell Carcinoma.Curr Oncol. 2025 Jun 7;32(6):335. doi: 10.3390/curroncol32060335. Curr Oncol. 2025. PMID: 40558278 Free PMC article.
References
-
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. 10.1002/ijc.29210. - PubMed
-
- Elanany MM, Mostafa D, Hamdy NM. Remodeled tumor immune microenvironment (TIME) parade via natural killer cells reprogramming in breast cancer. Life Sci. 2023;330:121997. 10.1016/j.lfs.2023.121997. - PubMed
-
- Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic Non-Small-Cell lung Cancer. N Engl J Med. 2018;378:2078–92. 10.1056/NEJMoa1801005. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

