Cold atmospheric plasma drives USP49/HDAC3 axis mediated ferroptosis as a novel therapeutic strategy in endometrial cancer via reinforcing lactylation dependent p53 expression
- PMID: 40234906
- PMCID: PMC11998187
- DOI: 10.1186/s12967-025-06449-8
Cold atmospheric plasma drives USP49/HDAC3 axis mediated ferroptosis as a novel therapeutic strategy in endometrial cancer via reinforcing lactylation dependent p53 expression
Abstract
Background: Endometrial cancer ranks among the most common gynecological cancers, with increasing rates of incidence and death. Cold atmospheric plasma (CAP) has become a promising novel therapeutic approach for cancer treatment. Nevertheless, the specific impact of CAP on endometrial cancer remains inadequately characterized.
Objectives: This study aimed to investigate the effect of CAP on the progression of endometrial cancer and reveal its specific regulatory mechanisms.
Methods: Colony formation, EdU, wound-healing, and transwell assay were used to detect the effect of CAP on endometrial cancer progression. Proteomics is employed to identify potential targets and signaling pathways through which CAP impacts endometrial cancer cells. MDA, lipid ROS, and JC-1 MMP assays were used to detect ferroptosis. Immunoprecipitation-mass spectrometry, co-immunoprecipitation, immunofluorescence co-localization, and molecular docking were used to analyze USP49 and HDAC3 interactions. The tumor xenografts model determined that CAP inhibits endometrial cancer growth in vivo.
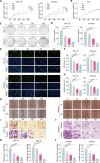
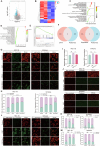
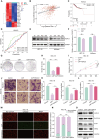
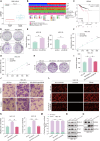
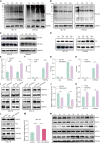
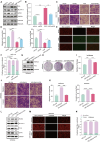
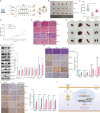
Results: This study observed a significant inhibitory effect of CAP on the proliferation and migration of endometrial cancer cells and reported for the first time that CAP induces ferroptosis in endometrial cancer cells. Mechanistically, CAP activated the transcription of p53 by modulating HDAC3 mediated the histone H3K18 lactylation, resulting in upregulation of p53 driving cell ferroptosis. The interaction between USP49 and HDAC3 was validated through mass spectrometry and co-immunoprecipitation experiments. The regulation of HDAC3 by CAP is contingent upon USP49, wherein the down-regulation of USP49 augments the ubiquitination of HDAC3, consequently diminishing its protein stability. Furthermore, animal models with transplanted tumors corroborated the inhibitory impact of CAP on endometrial cancer in vivo.
Conclusions: Our findings illustrate the suppressive effect of CAP treatment on endometrial cancer and uncover a novel regulatory mechanism mediated by CAP. Specifically, CAP modulates the ferroptosis pathway through the HDAC3/H3K18la/p53 axis, presenting a novel therapeutic approach for endometrial cancer treatment.
Keywords: CAP; Endometrial cancer; Ferroptosis; HDAC3; p53.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All aspects of this study were approved by the Ethics Committee of the Third Affiliated Hospital of Zhengzhou University (Approval Number: 2020-133-01 ). Consent for publication: All authors acknowledge and consent to the paper’s content and are included as co-authors. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Cold plasma irradiation inhibits skin cancer via ferroptosis.Biomed Phys Eng Express. 2024 Oct 10;10(6). doi: 10.1088/2057-1976/ad8200. Biomed Phys Eng Express. 2024. PMID: 39390682
-
SOX4 inhibits ferroptosis and promotes proliferation of endometrial cancer cells via the p53/SLC7A11 signaling.J Obstet Gynaecol Res. 2024 Nov;50(11):2093-2106. doi: 10.1111/jog.16094. Epub 2024 Sep 24. J Obstet Gynaecol Res. 2024. PMID: 39318043
-
Cold atmospheric plasma enhances SLC7A11-mediated ferroptosis in non-small cell lung cancer by regulating PCAF mediated HOXB9 acetylation.Redox Biol. 2024 Sep;75:103299. doi: 10.1016/j.redox.2024.103299. Epub 2024 Aug 3. Redox Biol. 2024. PMID: 39127016 Free PMC article.
-
Gingerenone A induces ferroptosis in colorectal cancer via targeting suppression of SLC7A11 signaling pathway.Biomed Pharmacother. 2024 Nov;180:117529. doi: 10.1016/j.biopha.2024.117529. Epub 2024 Oct 10. Biomed Pharmacother. 2024. PMID: 39393329
-
HDAC3: A Multifaceted Modulator in Immunotherapy Sensitization.Vaccines (Basel). 2025 Feb 13;13(2):182. doi: 10.3390/vaccines13020182. Vaccines (Basel). 2025. PMID: 40006729 Free PMC article. Review.
References
-
- Abu-Rustum N, Yashar C, Arend R, Barber E, Bradley K, Brooks R, Campos SM, Chino J, Chon HS, Chu C, et al. Uterine neoplasms, version 1.2023, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2023;21:181–209. - PubMed
MeSH terms
Substances
Grants and funding
- Grant No. 82173018/National Natural Science Foundation of China
- Grant No.232300421054, 232300421286/Natural Science Foundation of Henan Province
- Grant No. SBGJ202403034/the Medical Science and Technology Provincial and Ministerial Co-construction Project of Henan province
- Grant No. 242301420071/Henan Province Science and Technology Research and Development Joint Fund
- Grant No. YXKC2021036/the Young and Middle-aged Health Science and Technology Innovation Talents Project of Henan province
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

