Mitochondrial quality control in hematopoietic stem cells: mechanisms, implications, and therapeutic opportunities
- PMID: 40234908
- PMCID: PMC12001479
- DOI: 10.1186/s13287-025-04304-7
Mitochondrial quality control in hematopoietic stem cells: mechanisms, implications, and therapeutic opportunities
Abstract
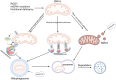
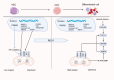
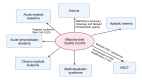
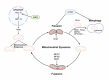
Mitochondrial quality control (MQC) is a critical mechanism for maintaining mitochondrial function and cellular metabolic homeostasis, playing an essential role in the self-renewal, differentiation, and long-term stability of hematopoietic stem cells (HSCs). Recent research highlights the central importance of MQC in HSC biology, particularly the roles of mitophagy, mitochondrial biogenesis, fission, fusion and mitochondrial transfer in regulating HSC function. Mitophagy ensures the removal of damaged mitochondria, maintaining low levels of reactive oxygen species (ROS) in HSCs, thereby preventing premature aging and functional decline. Concurrently, mitochondrial biogenesis adjusts key metabolic regulators such as mitochondrial transcription factor A (TFAM) and peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) to meet environmental demands, ensuring the metabolic needs of HSCs are met. Additionally, mitochondrial transfer, as an essential form of intercellular material exchange, facilitates the transfer of functional mitochondria from bone marrow stromal cells to HSCs, contributing to damage repair and metabolic support. Although existing studies have revealed the significance of MQC in maintaining HSC function, the precise molecular mechanisms and interactions among different regulatory pathways remain to be fully elucidated. Furthermore, the potential role of MQC dysfunction in hematopoietic disorders, including its involvement in disease progression and therapeutic resistance, is not yet fully understood. This review discusses the molecular mechanisms of MQC in HSCs, its functions under physiological and pathological conditions, and its potential therapeutic applications. By summarizing the current progress in this field, we aim to provide insights for further research and the development of innovative treatment strategies.
Keywords: Hematopoietic stem cell; Mitochondrial biogenesis; Mitochondrial dynamics; Mitochondrial metabolism; Mitochondrial quality control; Mitochondrial transfer; Mitophagy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no conflicts of interest.
Figures
Similar articles
-
Mitochondrial dynamics dysfunction and neurodevelopmental disorders: From pathological mechanisms to clinical translation.Neural Regen Res. 2025 Jun 19. doi: 10.4103/NRR.NRR-D-24-01422. Online ahead of print. Neural Regen Res. 2025. PMID: 40537021
-
Mitochondrial Quality Control in Health and Disease.MedComm (2020). 2025 Aug 15;6(8):e70319. doi: 10.1002/mco2.70319. eCollection 2025 Aug. MedComm (2020). 2025. PMID: 40821693 Free PMC article. Review.
-
Rho kinase isoforms in neurodegeneration: from cellular functions to therapeutic targets.Mol Biol Rep. 2025 Aug 26;52(1):846. doi: 10.1007/s11033-025-10947-9. Mol Biol Rep. 2025. PMID: 40856865 Review.
-
Role of mitochondria in physiological activities, diseases, and therapy.Mol Biomed. 2025 Jun 19;6(1):42. doi: 10.1186/s43556-025-00284-5. Mol Biomed. 2025. PMID: 40536597 Free PMC article. Review.
-
An emerging role of mitochondrial quality control in bone metabolism: from molecular mechanisms to targeted therapeutic interventions.Cell Mol Life Sci. 2025 Jul 29;82(1):291. doi: 10.1007/s00018-025-05802-w. Cell Mol Life Sci. 2025. PMID: 40728722 Free PMC article. Review.
Cited by
-
Targeting Mitochondrial Quality Control for the Treatment of Triple-Negative Breast Cancer: From Molecular Mechanisms to Precision Therapy.Biomolecules. 2025 Jul 5;15(7):970. doi: 10.3390/biom15070970. Biomolecules. 2025. PMID: 40723842 Free PMC article. Review.
-
Oxidative Stress and Mitochondrial Dysfunction in Myelodysplastic Syndrome: Roles in Development, Diagnosis, Prognosis, and Treatment.Int J Mol Sci. 2025 Jul 3;26(13):6415. doi: 10.3390/ijms26136415. Int J Mol Sci. 2025. PMID: 40650192 Free PMC article. Review.
References
-
- Fathi E, Ehsani A, Sanaat Z, Vandghanooni S, Farahzadi R, Montazersaheb S. Hematopoietic stem cells characteristics: from isolation to transplantation. Curr Stem Cell Res Ther. 2022;17(5):407–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

