Eggshell waste as a bioremoval agent for potentially toxic elements/metals and microbial contaminants from raw water of the Nile River in Egypt
- PMID: 40234932
- PMCID: PMC12001613
- DOI: 10.1186/s13104-025-07199-y
Eggshell waste as a bioremoval agent for potentially toxic elements/metals and microbial contaminants from raw water of the Nile River in Egypt
Abstract
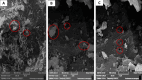
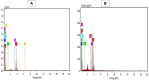
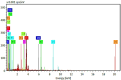
Recently, microbial, and potentially toxic elements/metals (PTEs) contamination of aquatic ecosystems has been increasing in Egypt, owing to the bio-disposal of such pollutants in water effluents. This study focused on using Eggshell waste (ESW) as a bioremoval agent for metals and microbial contaminants from raw water of the Nile river in Egypt which considered the source for life for all Egyptians. ESW was collected from local bakeries in Cairo, Egypt, and prepared for use as bioadsorbent. All raw water samples were treated with prepared ESW and tested for initial and end concentrations of PTEs and microbial load contents. Moreover, Scanning Electron Microscopy with Energy Dispersive X-ray analysis (SEM-EDX) was performed to test ESW characterization properties before and after raw water treatment using ESW. Results obtained by SEM recorded irregular rhombus-like stereo structures with tiny pore structures and lamellar structures with enlarged pore architectures dispersed randomly on the surface before ESW treatment. After ESW treatment, SEM-EDX results indicated a regular and adhesive appearance on the surface of ESW. Moreover, current results revealed that bioremoval efficiency reached 94.4, 64.7, and 51.4% for removing lead, cadmium, and iron, using ESW, respectively. Moreover, ESW was highly effective in eliminating Escherichia coli throughout the first 4 h of contacting and inhibiting 70% of the microbial load incubated at 37 °C, and complete inhibition occurred after 24 h of contacting process. Overall, this study advances knowledge in bioremediation and offers practical solutions for water quality management using organic waste materials.
Keywords: Bioremediation; Eggshell waste; Microbial contamination; Nile river; Potentially toxic elements/metals; Raw water.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This article does not contain any studies with human participants or animals performed by any of the authors. All the pathogenic bacteria were eradicated by autoclaving for 20 min. Competing interests: The authors declare no competing interests.
Figures



References
-
- Li B, Li LY, Grace JR. Adsorption and hydraulic conductivity of landfill-leachate perfluorinated compounds in bentonite barrier mixtures. J Environ Manage. 2015;156. 10.1016/j.jenvman.2015.04.003. - PubMed
-
- Singh V, Singh N, Rai SN, Kumar A, Singh AK, Singh MP, Sahoo A, Shekhar S, Vamanu E, Mishra V. 2023. Heavy Metal Contamination in the Aquatic Ecosystem: Toxicity and Its Remediation Using Eco-Friendly Approaches. Toxics, 11, 147. https://www.intechopen.com/chapters/82246#:~:text=DOI%3A%2010.5772/intec... - PMC - PubMed
-
- Mohod C, Dhote J. Review of heavy metals in drinking water and their effect on human health. Int J Innovative Res Sci Eng Technol. 2013;2:2992–6. https://www.researchgate.net/publication/251237189_Review_of_heavy_metal....
-
- Maity S, Bajirao Patil P, SenSharma S, Sarkar A. Bioremediation of heavy metals from the aqueous environment using Artocarpus heterophyllus (jackfruit) seed as a novel biosorbent. Chemosphere. 2022;307:136115. 10.1016/j.chemosphere.2022.136115. - PubMed
-
- Sankaran R, Show PL, Ooi C-W, Ling TC, Shu-Jen C, Chen S-Y, Chang Y-K. Feasibility assessment of removal of heavy metals and soluble microbial products from aqueous solutions using eggshell wastes. Clean Technol Environ Policy. 2020;22(4):773–86. 10.1007/s10098-019-01792-z.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

