Incidence and clinical outcomes of ventilator-associated events in Russian tertiary care settings: an analysis of electronic health records
- PMID: 40234949
- PMCID: PMC12001673
- DOI: 10.1186/s13104-025-07240-0
Incidence and clinical outcomes of ventilator-associated events in Russian tertiary care settings: an analysis of electronic health records
Abstract
Objective: This research aimed to evaluate the epidemiological and clinical characteristics of ventilator-associated events (VAE) using the CDC framework in a tertiary hospital in Moscow, Russia.
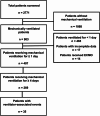
Results: In this cohort study, we analyzed electronic health records from 407 mechanically ventilated adults who were admitted to the Kommunarka Moscow Multipurpose Clinical Center between September 2022 and December 2023. We identified a total of 35 VAE, resulting in an incidence rate of 8.39 (95% confidence interval, 5.84 to 11.67) events per 1,000 ventilator-days. The presence of VAE was associated with higher ICU mortality by day 30 from the start of mechanical ventilation (adjusted hazard ratio, 1.58; 95% confidence interval, 1.01 to 2.48), particularly in patients with infection-related ventilator-associated complications (adjusted hazard ratio, 2.09; 95% confidence interval, 1.17 to 3.74). The median durations of mechanical ventilation and ICU length of stay were comparable between patients with VAE and those without. Implementing surveillance measures and developing tailored preventive strategies for VAE may be beneficial in similar healthcare settings to improve outcomes for mechanically ventilated patients.
Keywords: Epidemiology; Infection-related complication; Intensive care; Mechanical ventilation; Ventilator-associated event; Ventilator-associated pneumonia.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This research was conducted in accordance with the principles set forth in the Declaration of Helsinki. The study protocol received approval from the ethical committee of Kommunarka MMCC (protocol №6, dated September 24, 2024). Due to the retrospective nature of data collection, informed consent from individual participants was waved. Anonymization was utilized to ensure the confidentiality of all subjects involved. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- Klompas M. Ventilator-associated events: what they are and what they are not. Respir Care. 2019;64(8):953–61. - PubMed
-
- Stevens JP, Kachniarz B, Wright SB, Gillis J, Talmor D, Clardy P, et al. When policy gets it right: variability in U.S. Hospitals’ diagnosis of ventilator-associated pneumonia*. Crit Care Med. 2014;42(3):497–503. - PubMed
-
- Klompas M. Interobserver variability in ventilator-associated pneumonia surveillance. Am J Infect Control. 2010;38(3):237–9. - PubMed
-
- Skrupky LP, McConnell K, Dallas J, Kollef MH. A comparison of ventilator-associated pneumonia rates as identified according to the National healthcare safety network and American college of chest physicians criteria. Crit Care Med. 2012;40(1):281–4. - PubMed
-
- Centers for Disease Control and Prevention. Ventilator-association event (VAE). http://www.cdc.gov/nhsn/PDFs/pscManual/10-VAE_FINAL.pdf Accessed 5 Dec 2024.
MeSH terms
LinkOut - more resources
Full Text Sources

