The CentiMarker project: Standardizing quantitative Alzheimer's disease fluid biomarkers for biologic interpretation
- PMID: 40235082
- PMCID: PMC12000244
- DOI: 10.1002/alz.14587
The CentiMarker project: Standardizing quantitative Alzheimer's disease fluid biomarkers for biologic interpretation
Abstract
Introduction: Biomarkers play a crucial role in understanding Alzheimer's disease (AD) pathogenesis and treatment effects. However, comparing biomarker measures without standardization and appreciating their magnitude relative to the disease can be challenging.
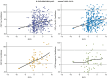
Methods: To address this issue, we propose the CentiMarker approach, similar to Centiloid, which provides a standardized scale between normal (0) and nearly maximum abnormal AD (100) ranges. We applied this scale to cerebrospinal fluid (CSF) biomarkers in dominantly inherited AD and sporadic AD cohorts.
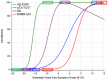
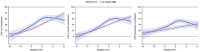
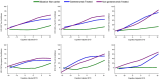
Results: CentiMarkers facilitated the interpretation of disease abnormality, demonstrating comparable changes and distributions of AD biomarkers across disease stages. CentiMarkers make the treatment effect more comparable than their original scales across various biomarkers.
Discussion: The versatility of CentiMarkers makes it a valuable tool for standardized biomarker comparison in AD research, enabling informed cross-study comparisons and contributing to accelerated therapeutic development. Adoption of the CentiMarker scale could enhance biomarker reporting and advance our understanding of AD.
Highlights: Comparing fluid biomarkers without appreciating their magnitude relative to the disease can be challenging. We propose a CentiMarker metric to standardize biomarker measures from normal (0) and nearly maximum abnormal AD (100) ranges. CentiMarkers make the treatment effect more comparable across various biomarkers than when using the original scales.
Keywords: Alzheimer's disease; CentiMarker; biomarker standardization; fluid biomarker.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
G.W.is the biostatistics core co‐leader for the Dominantly Inherited Alzheimer Network‐Trial Unit (DIAN‐TU). He discloses serving on the Data Safety Monitoring Board (DSMB) for Eli Lilly and Company, Amydis Corporate, and Abata Therapeutics, as well as working as a statistical consultant for Alector, Inc. and Pharmapace, Inc. He also serves as DSMB member for another five studies funded by the National Institutes of Health (NIH). R.J.B.is the Director of the DIAN‐TU and Principal Investigator of the DIAN‐TU‐001. He co‐founded C2N Diagnostics. Washington University and R.J.B. have equity ownership interest in C2N Diagnostics and receive royalty income based on technology (stable isotope labeling kinetics, blood plasma assay, and methods of diagnosing Alzheimer's disease [AD] with phosphorylation changes) that is licensed by Washington University to C2N Diagnostics. R.J.B. receives income from C2N Diagnostics for serving on the scientific advisory board. R.J.B. has received research funding from Avid Radiopharmaceuticals, Janssen, Roche/Genentech, Eli Lilly, Eisai, Biogen, AbbVie, Bristol Myers Squibb, and Novartis. He receives research support from the National Institute on Aging (NIA) of the NIH, DIAN‐TU Trial Pharmaceutical Partners (Eli Lilly and Company, F. Hoffman‐La Roche, Ltd., and Avid Radiopharmaceuticals), Alzheimer's Association, GHR Foundation, Anonymous Organization, DIAN‐TU Pharma Consortium (Active: Biogen, Eisai, Eli Lilly and Company, Janssen, F. Hoffmann‐La Roche, Ltd./Genentech. Previous: AbbVie, Amgen, AstraZeneca, Forum, Mithridion, Novartis, Pfizer, Sanofi, and United Neuroscience). He has been an invited speaker for Novartis and serves on the Advisory Board for F. Hoffman La Roche, Ltd. E.M.is the Associate Director of the DIAN‐TU. He reports serving on a Data Safety Committee for Eli Lilly and Company and Alector; scientific consultant for Eisai and Eli Lilly and Company; institutional grant support from Eli Lilly and Company, F. Hoffmann‐La Roche, Ltd., and Janssen. J.L.D. is an inventor on patents or patent applications of Eli Lilly and Company relating to the assays, methods, reagents and/or compositions of matter for p‐tau assays and Aβ‐targeting therapeutics. J.L.D. has served as a consultant on advisory boards for Eisai, Abbvie, Genotix Biotechnologies Inc, Gates Ventures, Karuna Therapeutics, AlzPath Inc., Cognito Therapeutics, Inc., and Prevail Therapeutics, and received research support from ADx Neurosciences, Fujirebio, AlzPath Inc., Roche Diagnostics, and Eli Lilly and Company in the past 2 years. J.L.D. has received speaker fees from Eli Lilly and Company. J.L.D. is a founder and advisor for Monument Biosciences. J.L.D. has stock or stock options in Eli Lilly and Company, Genotix Biotechnologies, AlzPath Inc., and Monument Biosciences. S.E.S. has served as a consultant or an advisory board or received speaker's fees from Eisai, Eli Lilly, and Novo Nordisk. K.B. has served as a consultant and at advisory boards for Abbvie, AC Immune, ALZPath, AriBio, BioArctic, Biogen, Eisai, Lilly, Moleac Pte. Ltd, Neurimmune, Novartis, Ono Pharma, Prothena, Roche Diagnostics, and Siemens Healthineers; has served on data monitoring committees for Julius Clinical and Novartis; has given lectures, produced educational materials, and participated in educational programs for AC Immune, Biogen, Celdara Medical, Eisai, and Roche Diagnostics; and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this article. H.Z. has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, LabCorp, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave; has given lectures in symposia sponsored by Alzecure, Biogen, Cellectricon, Fujirebio, Lilly, Novo Nordisk, and Roche; and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside the submitted work). C.C. has received research support from GSK and Eisai. The funders of the study had no role in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. C.C. is a member of the advisory board of Circular Genomics and owns stocks in this company. All the other authors reported no conflicts of interest. Author disclosures are available in the Supporting Information.
Figures



Update of
-
The CentiMarker Project: Standardizing Quantitative Alzheimer's disease Fluid Biomarkers for Biologic Interpretation.medRxiv [Preprint]. 2024 Jul 27:2024.07.25.24311002. doi: 10.1101/2024.07.25.24311002. medRxiv. 2024. Update in: Alzheimers Dement. 2025 Apr;21(4):e14587. doi: 10.1002/alz.14587. PMID: 39108526 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
- Korea Health Technology R&D
- German Center for Neurodegenerative Diseases (DZNE)
- Alzheimer's Disease Neuroimaging Initiative
- F. Hoffman-LaRoche Ltd.
- U01 AG024904/AG/NIA NIH HHS/United States
- ALZ/Alzheimer's Association/United States
- U01 AG052564/AG/NIA NIH HHS/United States
- Raul Carrea Institute for Neurological Research (FLENI)
- Korea Health Industry Development Institute (KHIDI)
- R01 AG053267/AG/NIA NIH HHS/United States
- AbbVie, Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd
- Japan Agency for Medical Research and Development (AMED)
- National Institute on Aging (NIA)
- The Dominantly Inherited Alzheimer Network
- R01AG046179/FNIH and Accelerating Medicines Partnership
- Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research
- U01 AG024904/NH/NIH HHS/United States
- U01AG052564/Foundation for the National Institutes of Health
- R01 AG046179/AG/NIA NIH HHS/United States
- Korea Dementia Research Center (KDRC)
- GHR Foundation
- HI21C0066/the Ministry of Health & Welfare and Ministry of Science and ICT, Republic of Korea
- R01AG053267-S1/FNIH and Accelerating Medicines Partnership
- EB/NIBIB NIH HHS/United States
- Avid Radiopharmaceuticals
- Eli Lilly and Company
- U01AG042791-S1/NH/NIH HHS/United States
- Spanish Institute of Health Carlos III (ISCIII)
- U19 AG032438/AG/NIA NIH HHS/United States
- W81XWH-12-2-0012/Department of Defense
- U01 AG042791/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

