Alterations in dopaminergic innervation and receptors in focal cortical dysplasia
- PMID: 40235315
- PMCID: PMC12316006
- DOI: 10.1093/brain/awaf080
Alterations in dopaminergic innervation and receptors in focal cortical dysplasia
Abstract
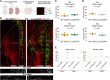
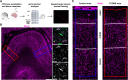
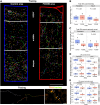
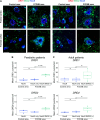
Focal cortical dysplasia (FCD) type 2 is the most common malformation of cortical development associated with pharmaco-resistant focal epilepsy and frequently located in the frontal cortex. Neuropathological hallmarks comprise abnormal cortical layering and enlarged, dysmorphic neuronal elements. Fundamentally altered local neuronal activity has been reported in human FCD type 2 epilepsy surgical biopsies. Of note, FCD type 2 emerges during brain development and forms complex connectivity architectures with surrounding neuronal networks. Local cortical microcircuits, particularly in frontal localization, are extensively modulated by monoaminergic axonal projections originating from the brainstem. Previous analysis of monoaminergic modulatory inputs in human FCD type 2 biopsies suggested altered density and distribution of these monoaminergic axons; however, a systematic investigation is still pending. Here, we perform a comprehensive analysis of dopaminergic (DA) innervation, in human FCD type 2 biopsies and in the medial prefrontal cortex (mPFC) of an FCD type 2 mouse model [mechanistic target of rapamyin (mTOR) hyperactivation model] during adolescent and adult stages. In addition, we analyse the expression of dopamine receptor transcripts via multiplex fluorescent RNA in situ hybridization in human specimens and the mPFC of this mouse model. In the mTOR hyperactivation mouse model, we observe a transient alteration of DA innervation density during adolescence and a trend towards decreased innervation in adulthood. In human FCD type 2 areas, the overall DA innervation density is decreased in adult patients compared with control areas from these patients. Moreover, the DA innervation shows an altered lamination pattern in the FCD type 2 area compared with the control area. Dopamine receptors 1 and 2 appear to be differentially expressed in the dysmorphic neurons in human samples and mTOR-mutant cells in mice compared with normally developed neurons. Intriguingly, our results suggest complex molecular and structural alterations putatively inducing impaired DA neurotransmission in FCD type 2. We hypothesize that this may have important implications for the development of these malformations and the manifestation of seizures.
Keywords: cortical malformations; epileptogenesis; neurodevelopmental disorders; neuromodulation; neurotransmitter systems.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures

References
-
- Auvin S, Baulac S. mTOR-therapy and targeted treatment opportunities in mTOR-related epilepsies associated with cortical malformations. Rev Neurol (Paris). 2023;179:337–344. - PubMed
-
- Cepeda C, Hurst RS, Flores-Hernández J, et al. Morphological and electrophysiological characterization of abnormal cell types in pediatric cortical dysplasia. J Neurosci Res. 2003;72:472–486. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

