First-in-human study of CPL'116 - a dual JAK/ROCK inhibitor - in healthy subjects
- PMID: 40235539
- PMCID: PMC11996792
- DOI: 10.3389/fphar.2025.1583723
First-in-human study of CPL'116 - a dual JAK/ROCK inhibitor - in healthy subjects
Abstract
Background: CPL'116 is a novel Janus kinase (JAK) and Rho-associated coiled-coil containing protein kinase (ROCK) dual inhibitor and a promising drug candidate for the treatment of inflammatory and fibrotic diseases. We conducted this first-in-human, Phase I clinical trial to evaluate the safety, pharmacokinetics (PK), and exploratory pharmacodynamics (PD) of CPL'116 in healthy subjects.
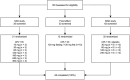
Methods: Phase I clinical trial in healthy White volunteers was conducted after single (n = 21, 10-300 mg) and multiple (n = 32, 30-240 mg or placebo, 14-day b.i.d.) administrations of CPL'116 including a food effect study (n = 12, 120 mg). The multiple ascending dose part was double-blinded and placebo-controlled. The primary endpoint was safety evaluation, and the secondary endpoint was PK. Exploratory PD was studied by measuring the inhibition of JAK and ROCK in the blood by assessing STAT1, STAT5, and MLC phosphorylation.
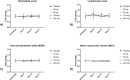
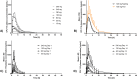
Results: Safety parameters were comparable between the placebo and active treatment groups, with no clinically meaningful variations in the safety parameters between the cohorts. No deaths or serious adverse events (SAEs) were reported. No influence on hematological parameters (neutrophil count, red cell distribution width, and mean corpuscular volume) was observed. Plasma Cmax and AUC increased proportionally in the dosing range of 60-240 mg. Median tmax ranged 2-3 h. Food increased the absorption of CPL'116. Compared to placebo, CPL'116 at 240 mg dose showed a decrease in the phosphorylation of STAT1 (Days 1 and 14, p < 0.05) and STAT5 (Day 14, p < 0.05). A decrease in MLC phosphorylation indicated a potential trend at p < 0.1.
Conclusion: CPL'116 was safe and well-tolerated by healthy subjects. The PK profile is well suited for twice-daily administration and justifies further clinical development. Exploratory PD studies indicated the ability of CPL'116 to affect the JAK and ROCK pathways in humans, hinting at its potential therapeutic role in diseases benefiting from its dual mode of action. The positive results of this study indicate the possibility of developing a novel class of therapeutics that address both inflammatory and fibrotic processes.
Clinical trial registrationmethods: clinicaltrials.gov, identifier NCT04670757.
Keywords: CPL’116 (previously CPL409116); Janus kinase (JAK); Rho kinase (ROCK); inflammation; pharmacodynamics; pharmacokinetics; phase I (drug development); safety.
Copyright © 2025 Rudzki, Jarus-Dziedzic, Włodarczyk, Kaza, Pankiewicz, Gierczak-Pachulska, Banach, Zygmunt, Piwowarczyk, Żero, Rabczenko, Segiet-Święcicka and Wieczorek.
Conflict of interest statement
PJR, DW, MK, PP, AG-P, MB, CP, BZ, and PŻ were full-time employees of Celon Pharma S.A. and may declare stock ownership. MW is a founder, CEO, and majority owner of Celon Pharma S.A. KJ-D was a contracted principal investigator for this study and sub-investigator in clinical studies of other JAK inhibitors contracted by pharmaceutical companies. DR and AS-Ś are paid consultants (statisticians) for Celon Pharma S.A.
Figures

Similar articles
-
Randomized, double-blinded, placebo-controlled phase I study of the pharmacokinetics, pharmacodynamics, and safety of KL130008, a novel oral JAK inhibitor, in healthy subjects.Eur J Pharm Sci. 2022 Sep 1;176:106257. doi: 10.1016/j.ejps.2022.106257. Epub 2022 Jul 9. Eur J Pharm Sci. 2022. PMID: 35820629 Clinical Trial.
-
A new fully human recombinant FSH (follitropin epsilon): two phase I randomized placebo and comparator-controlled pharmacokinetic and pharmacodynamic trials.Hum Reprod. 2017 Aug 1;32(8):1639-1647. doi: 10.1093/humrep/dex220. Hum Reprod. 2017. PMID: 28591833 Clinical Trial.
-
Pharmacokinetic characteristics of golidocitinib, a highly selective JAK1 inhibitor, in healthy adult participants.Front Immunol. 2023 Apr 3;14:1127935. doi: 10.3389/fimmu.2023.1127935. eCollection 2023. Front Immunol. 2023. PMID: 37077916 Free PMC article.
-
A Phase I, Randomized, Double-Blind, Placebo-Controlled, Single Ascending Dose, Multiple Dose, and Food Effect Trial of the Safety, Tolerability and Pharmacokinetics of Highly Purified Cannabidiol in Healthy Subjects.CNS Drugs. 2018 Nov;32(11):1053-1067. doi: 10.1007/s40263-018-0578-5. CNS Drugs. 2018. PMID: 30374683 Free PMC article. Clinical Trial.
-
Oral Janus kinase inhibitors for maintenance of remission in ulcerative colitis.Cochrane Database Syst Rev. 2020 Jan 27;1(1):CD012381. doi: 10.1002/14651858.CD012381.pub2. Cochrane Database Syst Rev. 2020. PMID: 31984480 Free PMC article.
References
-
- Cai W., Tong R., Sun Y., Yao Y., Zhang J. (2024). Comparative efficacy of five approved Janus kinase inhibitors as Monotherapy and Combination Therapy in patients with moderate-to-severe active rheumatoid arthritis: a Systematic review and Network Meta-analysis of randomized controlled trials. Front. Pharmacol. 15 (April), 1387585. 10.3389/fphar.2024.1387585 - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

