Natural compounds: new therapeutic approach for inhibition of Streptococcus mutans and dental caries
- PMID: 40235544
- PMCID: PMC11996897
- DOI: 10.3389/fphar.2025.1548117
Natural compounds: new therapeutic approach for inhibition of Streptococcus mutans and dental caries
Abstract
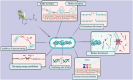
Streptococcus mutans is recognized as one of the leading causes of dental caries, and biofilm formation by this bacterium plays a key role in dental plaque development and caries progression. Given the increasing resistance of bacteria to antibiotics and the adverse effects of some synthetic antimicrobials, the search for natural alternatives has received increasing attention. The recently published studies have demonstrated that natural compounds (NCs) such as curcumin, cinnamaldehyde, eugenol, thymol, carvacrol, epigallocatechin gallate, farnesol, catechin, inulin, menthol, apigenin, myricetin, oleanolic acid, and resveratrol, have notable antimicrobial properties and can effectively inhibit the growth of Streptococcus mutans. NCs can disrupt bacterial membrane integrity, leading to cell death, and possess the capability to inhibit acid production, which is a key factor in caries development. NCs can also interfere with bacterial adhesion to surfaces, including teeth. The attachment inhibition is achieved by decreasing the expression of adhesion factors such as gtfs, ftf, fruA, and gbpB. NCs can disrupt bacterial metabolism, inhibit biofilm formation, disperse existing biofilm, and interfere with quorum sensing and two-component signal transduction systems. Moreover, novel drug delivery platforms were used to enhance the bioavailability and stability of NCs. Studies have also indicated that NCs exhibit significant efficacy in combination therapies. Notably, curcumin has shown promising results in photodynamic therapy against S. mutans. The current review article analyzes the mechanisms of action of various NCs against S. mutans and investigates their potential as alternative or complementary therapeutic options for managing this bacterium and dental caries.
Keywords: Streptococcus mutans; biofilm; dental caries; natural compounds; new treatment.
Copyright © 2025 Kashi, Varseh, Hariri, Chegini and Shariati.
Conflict of interest statement
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
Similar articles
-
The enhancing antibiofilm activity of curcumin on Streptococcus mutans strains from severe early childhood caries.BMC Microbiol. 2020 Sep 16;20(1):286. doi: 10.1186/s12866-020-01975-5. BMC Microbiol. 2020. PMID: 32938379 Free PMC article.
-
Eugenol-induced suppression of biofilm-forming genes in Streptococcus mutans: An approach to inhibit biofilms.J Glob Antimicrob Resist. 2014 Dec;2(4):286-292. doi: 10.1016/j.jgar.2014.05.006. Epub 2014 Jul 6. J Glob Antimicrob Resist. 2014. PMID: 27873689
-
Inhibition of Streptococcus mutans biofilm formation, extracellular polysaccharide production, and virulence by an oxazole derivative.Appl Microbiol Biotechnol. 2016 Jan;100(2):857-67. doi: 10.1007/s00253-015-7092-1. Epub 2015 Nov 3. Appl Microbiol Biotechnol. 2016. PMID: 26526453
-
New strategies and mechanisms for targeting Streptococcus mutans biofilm formation to prevent dental caries: A review.Microbiol Res. 2023 Oct 14;278:127526. doi: 10.1016/j.micres.2023.127526. Online ahead of print. Microbiol Res. 2023. PMID: 39491258 Review.
-
Natural compounds in the fight against Staphylococcus aureus biofilms: a review of antibiofilm strategies.Front Pharmacol. 2024 Nov 20;15:1491363. doi: 10.3389/fphar.2024.1491363. eCollection 2024. Front Pharmacol. 2024. PMID: 39635434 Free PMC article. Review.
Cited by
-
Formulation and Evaluation of a Licorice-Resveratrol Lollipop for Targeting Streptococcus mutans Biofilm and Antimicrobial Resistance.Infect Drug Resist. 2025 Aug 7;18:3933-3946. doi: 10.2147/IDR.S537534. eCollection 2025. Infect Drug Resist. 2025. PMID: 40792117 Free PMC article.
-
Natural compounds for colistin-resistant Acinetobacter baumannii biofilm control: eugenol, cinnamaldehyde, and carvacrol.Mol Biol Rep. 2025 Jul 18;52(1):735. doi: 10.1007/s11033-025-10844-1. Mol Biol Rep. 2025. PMID: 40679553 No abstract available.
-
The Role of Plant Oils in Combating Streptococcal Infections: Mechanisms, Efficacy, and Therapeutic Potential.Curr Microbiol. 2025 Aug 18;82(10):460. doi: 10.1007/s00284-025-04438-0. Curr Microbiol. 2025. PMID: 40824426 Review.
References
Publication types
LinkOut - more resources
Full Text Sources

