Use of mobile technology for reporting the pharmacovigilance of vaccines in Panama
- PMID: 40235580
- PMCID: PMC11997413
- DOI: 10.1016/j.pmedr.2025.103056
Use of mobile technology for reporting the pharmacovigilance of vaccines in Panama
Abstract
Objective: Monitoring adverse reactions is essential to confirm vaccine safety profiles. Studies using electronic tools for data collection may reach a broader audience, improving data efficiency and integrity, reducing study costs and simplifying data collection compared with nonelectronic methods. This study aimed to validate electronic versus paper diaries for reporting postimmunization reactions in Panama.
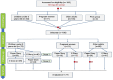
Methods: An experimental design was conducted with three groups (children, pregnant women, and older adults). Groups were divided into one subgroup using paper diary and one using electronic diary. Diary assignments were subsequently reversed in children group, which parents completed. Symptoms and reporting frequency were collected in 2020 and 2021. Information reported in paper diaries was entered into an electronic case report form and reconciled. Users' adherence, differences between reported symptom frequency and users' acceptability of diaries were evaluated.
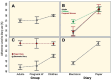
Results: A total of 180 participants were included: 79 children, 21 pregnant women, 80 older adults. Children group showed greater adherence to both diaries. No significant differences were found in response times in the electronic diary between groups. More symptoms were reported in the electronic diary. The experience of using diaries, no matter which one, was similar.
Conclusions: Results indicate young people adapt better to technological tools than older adults, suggesting tools should be adjusted according to the user's age. Furthermore, electronic applications for reporting postimmunization reactions offer suitable pharmacovigilance alternatives, providing real-time information, and requiring fewer staff, leading to improved health outcomes, patient compliance, and data for research and public health analysis, supporting global vaccine development.
Keywords: Mobile applications; Mobile technology; Panama; Pharmacovigilance; Vaccines.
© 2025 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Xavier Saez-Llorens reports a relationship with Secretaría Nacional de Ciencia, Tecnología e Innovación that includes: consulting or advisory. Rodrigo DeAntonio reports a relationship with Secretaría Nacional de Ciencia, Tecnología e Innovación that includes: consulting or advisory. This project was supported by grant number FID18–068. The Secretaria Nacional de Ciencia, Tecnología e Innovación, SENACYT, funded the study as part of the Public Call for Investigation and Research Promotion 2018, Republic of Panama. https://www.senacyt.gob.pa/. Likewise, Cevaxin provided its own resources to conduct this project. XSL, and RDA received the grant. The funders had no role in study design, data collection and analysis, the decision to publish, or the preparation of the manuscript. At the time of the study, RDA was part of the SENACYT investigators and received a salary at the time. XSL receives a salary from SENACYT and is part of their investigators. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Aherkar Rekha Y., Deshpande Pradeep K., Ghongane Balasaheb B. Study of the pattern of adverse events following immunization of children in a tertiary care hospital. Int. J. Basic Clin. Pharmacol. 2016:609–615.
-
- Branch, Statistics on Panama's Digital Situation in Estadísticas de la Situación Digital de PANAMÁ en el 2024. 2024. https://branch.com.co/marketing-digital/estadisticas-de-la-situacion-dig... Last accessed on March 2025.
LinkOut - more resources
Full Text Sources

