Hippocampal and cortical high-frequency oscillations orchestrate human semantic networks during word list memory
- PMID: 40235588
- PMCID: PMC11999489
- DOI: 10.1016/j.isci.2025.112171
Hippocampal and cortical high-frequency oscillations orchestrate human semantic networks during word list memory
Abstract
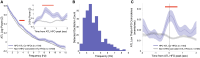
Episodic memory requires the precise coordination between the hippocampus and distributed cortical regions. This may be facilitated by bursts of brain activity called high-frequency oscillations (HFOs). We hypothesized that HFOs activate specific networks during memory retrieval and aimed to describe the electrophysiological properties of HFO-associated activity. To study this, we recorded intracranial electroencephalography while human participants performed a list learning task. Hippocampal HFOs (hHFOs) increased during encoding and retrieval, and these increases correlated with memory performance. During retrieval, hHFOs demonstrated activation of semantic processing regions that were previously active during encoding. This consisted of broadband high-frequency activity (HFA) and cortical HFOs. HFOs in the anterior temporal lobe, a major semantic hub, co-occurred with hHFOs, particularly during retrieval. These coincident HFOs were associated with greater cortical HFA and cortical theta bursts. Hence, HFOs may support synchronization of activity across distributed nodes of the hippocampal-cortical memory network.
Keywords: Cognitive neuroscience; Linguistics.
© 2025 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Longitudinal Differences in Human Hippocampal Connectivity During Episodic Memory Processing.Cereb Cortex Commun. 2020;1(1):tgaa010. doi: 10.1093/texcom/tgaa010. Epub 2020 Apr 14. Cereb Cortex Commun. 2020. PMID: 32864613 Free PMC article.
-
Theta-associated high-frequency oscillations (110-160Hz) in the hippocampus and neocortex.Prog Neurobiol. 2013 Jan;100:1-14. doi: 10.1016/j.pneurobio.2012.09.002. Epub 2012 Sep 25. Prog Neurobiol. 2013. PMID: 23022096 Review.
-
Hippocampal Theta Oscillations Support Successful Associative Memory Formation.J Neurosci. 2020 Dec 2;40(49):9507-9518. doi: 10.1523/JNEUROSCI.0767-20.2020. Epub 2020 Nov 6. J Neurosci. 2020. PMID: 33158958 Free PMC article.
-
Hippocampal Theta and Episodic Memory.J Neurosci. 2023 Jan 25;43(4):613-620. doi: 10.1523/JNEUROSCI.1045-22.2022. Epub 2022 Dec 8. J Neurosci. 2023. PMID: 36639900 Free PMC article.
-
Update on the mechanisms and roles of high-frequency oscillations in seizures and epileptic disorders.Epilepsia. 2017 Aug;58(8):1330-1339. doi: 10.1111/epi.13830. Epub 2017 Jul 6. Epilepsia. 2017. PMID: 28681378 Free PMC article. Review.
Cited by
-
Motifs of human high-frequency oscillations structure processing and memory of continuous audiovisual narratives.Sci Adv. 2025 Jul 25;11(30):eadv0986. doi: 10.1126/sciadv.adv0986. Epub 2025 Jul 25. Sci Adv. 2025. PMID: 40712018 Free PMC article.
References
LinkOut - more resources
Full Text Sources

