Early Growth Response Gene 1 Benefits Autoimmune Disease by Promoting Regulatory T Cell Differentiation as a Regulator of Foxp3
- PMID: 40235598
- PMCID: PMC11997311
- DOI: 10.34133/research.0662
Early Growth Response Gene 1 Benefits Autoimmune Disease by Promoting Regulatory T Cell Differentiation as a Regulator of Foxp3
Abstract
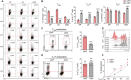
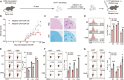
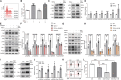
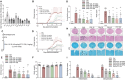
Foxp3+ regulatory T (Treg) cells, as one of the subtypes of CD4+ T cells, are the crucial gatekeeper in the pathogenesis of self-antigen reactive diseases. In this context, we demonstrated that the selective ablation of early growth response gene 1 (Egr-1) in CD4+ T cells exacerbated experimental autoimmune encephalomyelitis (EAE) in murine models. The absence of Egr-1 in CD4+ T cells, obtained from EAE mice and naïve CD4+ T cells, impeded the differentiation and influence of Treg. Importantly, in CD4+ T cells of multiple sclerosis patients, both Egr-1 and Foxp3 were found to decrease. Further studies showed that distinct from the classical Smad3 route, TGF-β could activate Egr-1 through the Raf-Erk signaling route to promote Foxp3 genetic modulation, thereby promoting Treg cell differentiation and reducing EAE inflammation. A novel natural Egr-1 agonist, calycosin, was found to attenuate EAE progression by regulating the differentiation of Treg. Together, the above results indicate the value of Egr-1, as a novel Foxp3 transactivator, for the differentiation of Treg cells in the development of self-antigen reactive diseases.
Copyright © 2025 Liu Yang et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures




Similar articles
-
Primaquine elicits Foxp3+ regulatory T cells with a superior ability to limit CNS autoimmune inflammation.J Autoimmun. 2020 Nov;114:102505. doi: 10.1016/j.jaut.2020.102505. Epub 2020 Jun 25. J Autoimmun. 2020. PMID: 32595012
-
S3I-201, a selective stat3 inhibitor, ameliorates clinical symptoms in a mouse model of experimental autoimmune encephalomyelitis through the regulation of multiple intracellular signalling in Th1, Th17, and treg cells.Mult Scler Relat Disord. 2023 May;73:104658. doi: 10.1016/j.msard.2023.104658. Epub 2023 Mar 23. Mult Scler Relat Disord. 2023. PMID: 36989705
-
Exosomes with FOXP3 from gene-modified dendritic cells ameliorate the development of EAE by regulating the balance of Th/Treg.Int J Med Sci. 2022 Jul 11;19(8):1265-1274. doi: 10.7150/ijms.72655. eCollection 2022. Int J Med Sci. 2022. PMID: 35928722 Free PMC article.
-
Therapeutic role of interferon-γ in experimental autoimmune encephalomyelitis is mediated through a tolerogenic subset of splenic CD11b+ myeloid cells.J Neuroinflammation. 2024 May 31;21(1):144. doi: 10.1186/s12974-024-03126-3. J Neuroinflammation. 2024. PMID: 38822334 Free PMC article.
-
Green tea EGCG, T cells, and T cell-mediated autoimmune diseases.Mol Aspects Med. 2012 Feb;33(1):107-18. doi: 10.1016/j.mam.2011.10.001. Epub 2011 Oct 14. Mol Aspects Med. 2012. PMID: 22020144 Review.
References
-
- Ben-Nun A, Kaushansky N, Kawakami N, Krishnamoorthy G, Berer K, Liblau R, Hohlfeld R, Wekerle H. From classic to spontaneous and humanized models of multiple sclerosis: Impact on understanding pathogenesis and drug development. J Autoimmun. 2014;54:33–50. - PubMed
-
- Oh J, Vidal-Jordana A, Montalban X. Multiple sclerosis: Clinical aspects. Curr Opin Neurol. 2018;31(6):752–759. - PubMed
-
- Kohm AP, Carpentier PA, Miller SD. Regulation of experimental autoimmune encephalomyelitis (EAE) by CD4+CD25+ regulatory T cells. Novartis Found Symp. 2003;252:45–52. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

