Chronic motoneuronal activation enhanced axonal regeneration and functional recovery after brachial plexus injury
- PMID: 40235635
- PMCID: PMC11999476
- DOI: 10.1016/j.jot.2025.02.007
Chronic motoneuronal activation enhanced axonal regeneration and functional recovery after brachial plexus injury
Abstract
Background: Brachial plexus injury (BPI) leads to significant impairment of upper limb motor function, primarily due to progressive atrophy of denervated muscles resulting from the slow rate of axonal regeneration. Therefore, identifying strategies to accelerate axon extension is of critical importance.
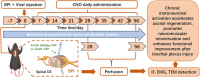
Methods: In this study, we first established a mouse model of brachial plexus injury and employed chemogenetic approaches to specifically activate C6 spinal motoneurons. We then assessed axonal regeneration and motor function recovery in the injured mice through behavioral tests, morphological analyses, and electrophysiological detection.
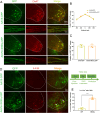
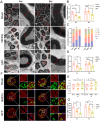
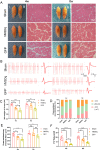
Results: We found that the AAV9-hM3Dq virus efficiently transduced motoneurons, and CNO administration robustly activated mature hM3Dq+ motoneurons in vivo. Chronic chemogenetic activation significantly enhanced the regeneration of spinal motoneurons injured by ventral root crush, accelerated axon extension, and improved axonal remyelination, resulting in increased axon size. This activation also facilitated the formation of new neuromuscular junctions (NMJs) in adult motoneurons and reduced muscle atrophy. Furthermore, it promoted electrophysiological recovery of the motor unit and improved overall motor function.
Conclusion: Chemogenetic activation of adult motoneurons can robustly enhances axon growth and mediate better behavioral recovery. These findings highlight the therapeutic potential of chemogenetic neuronal activation in promoting functional recovery following nerve injury.
The translational potential of this article: We have established a chronic chemogenetic method to activate hM3Dq+ motor neurons after brachial plexus injury, which accelerates axonal regeneration and enhances functional recovery. This strategy holds promise as a clinical therapeutic approach for treating nervous system injuries.
Keywords: Brachial plexus injury; Chemogenetic activation; Motor repair; Spinal motoneurons.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Lihua Zhou reports financial support was provided by Sun Yat-Sen University. Ke Zhong reports financial support was provided by Sun Yat-Sen Memorial Hospital. Reports a relationship with that includes:. Has patent pending to. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





Similar articles
-
Berberine enhances survival and axonal regeneration of motoneurons following spinal root avulsion and re-implantation in rats.Free Radic Biol Med. 2019 Nov 1;143:454-470. doi: 10.1016/j.freeradbiomed.2019.08.029. Epub 2019 Aug 28. Free Radic Biol Med. 2019. PMID: 31472247
-
LINGO-1 shRNA Loaded by Pluronic F-127 Promotes Functional Recovery After Ventral Root Avulsion.Tissue Eng Part A. 2019 Oct;25(19-20):1381-1395. doi: 10.1089/ten.TEA.2018.0282. Epub 2019 May 2. Tissue Eng Part A. 2019. PMID: 30794055
-
Acetylglutamine facilitates motor recovery and alleviates neuropathic pain after brachial plexus root avulsion in rats.J Transl Med. 2023 Aug 23;21(1):563. doi: 10.1186/s12967-023-04399-7. J Transl Med. 2023. PMID: 37612586 Free PMC article.
-
Therapeutic strategies for brachial plexus injury.Folia Neuropathol. 2021;59(4):393-402. doi: 10.5114/fn.2021.111996. Folia Neuropathol. 2021. PMID: 35114780 Review.
-
Prospect of Stem Cells as Promising Therapy for Brachial Plexus Injury: A Systematic Review.Stem Cells Cloning. 2022 Jun 22;15:29-42. doi: 10.2147/SCCAA.S363415. eCollection 2022. Stem Cells Cloning. 2022. PMID: 35770243 Free PMC article. Review.
Cited by
-
"Multidisciplinary synergy driving innovation in orthopaedic translational medicine".J Orthop Translat. 2025 Jun 4;52:A1-A3. doi: 10.1016/j.jot.2025.06.001. eCollection 2025 May. J Orthop Translat. 2025. PMID: 40698066 Free PMC article. No abstract available.
References
-
- Scheib J., Hoke A. Advances in peripheral nerve regeneration. Nat Rev Neurol. 2013;9(12):668–676. - PubMed
-
- Rasulić L., Savić A., Živković B., Vitošević F., Mićović M., Baščarević V., et al. Outcome after brachial plexus injury surgery and impact on quality of life. Acta Neurochir. 2017;159(7):1257–1264. - PubMed
-
- He Z., Jin Y. Intrinsic control of axon regeneration. Neuron. 2016;90(3):437–451. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

