Extracellular vesicle-LncRNA HOTAIR modulates esophageal cancer chemoresistance and immune microenvironment via miR-375/CDH2 pathway
- PMID: 40235720
- PMCID: PMC11996620
- DOI: 10.1002/ccs3.70014
Extracellular vesicle-LncRNA HOTAIR modulates esophageal cancer chemoresistance and immune microenvironment via miR-375/CDH2 pathway
Abstract
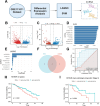
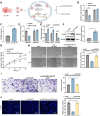
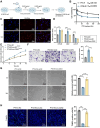
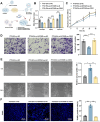
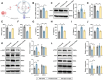
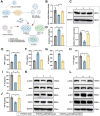
Chemoresistance and immune evasion remain significant barriers to effective esophageal cancer (EC) treatment. This study explores the mechanistic role of extracellular vesicles (EVs) delivering LncRNA HOTAIR in modulating these processes. Using transcriptomic profiling, LncRNA HOTAIR was identified as a critical factor in EC progression. Its interaction with miR-375 was examined via luciferase reporter assays and RNA immunoprecipitation. Paclitaxel-resistant EC cells were treated with EVs containing HOTAIR, and the functional impact on proliferation, migration, invasion, and immune response was assessed through in vitro and in vivo models. LncRNA HOTAIR in EVs enhanced paclitaxel resistance by suppressing miR-375 and increasing CDH2 expression. Furthermore, HOTAIR promoted immune escape by upregulating PD-L1, impairing T-cell-mediated cytotoxicity. These changes were validated in patient-derived EC models. This study demonstrates that EV-LncRNA HOTAIR mediates chemoresistance and immune evasion in EC by targeting the miR-375/CDH2 axis. These findings provide a foundation for novel therapeutic interventions targeting EV-HOTAIR.
Keywords: CDH2; LncRNA HOTAIR; chemoresistance; esophageal cancer; extracellular vesicles; immune evasion; miR‐375.
© 2025 The Author(s). Journal of Cell Communication and Signaling published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Long non-coding RNA HOTAIR functions as miRNA sponge to promote the epithelial to mesenchymal transition in esophageal cancer.Biomed Pharmacother. 2017 Jun;90:888-896. doi: 10.1016/j.biopha.2017.03.103. Epub 2017 Apr 22. Biomed Pharmacother. 2017. PMID: 28441714
-
LncRNA HOTAIR-mediated MTHFR methylation inhibits 5-fluorouracil sensitivity in esophageal cancer cells.J Exp Clin Cancer Res. 2020 Jul 11;39(1):131. doi: 10.1186/s13046-020-01610-1. J Exp Clin Cancer Res. 2020. PMID: 32653028 Free PMC article.
-
Long noncoding RNA HOTAIR mediates the estrogen-induced metastasis of endometrial cancer cells via the miR-646/NPM1 axis.Am J Physiol Cell Physiol. 2018 Jun 1;314(6):C690-C701. doi: 10.1152/ajpcell.00222.2017. Epub 2018 Feb 21. Am J Physiol Cell Physiol. 2018. PMID: 29466670
-
LncRNA HOTAIR is a Prognostic Biomarker for the Proliferation and Chemoresistance of Colorectal Cancer via MiR-203a-3p-Mediated Wnt/ß-Catenin Signaling Pathway.Cell Physiol Biochem. 2018;46(3):1275-1285. doi: 10.1159/000489110. Epub 2018 Apr 16. Cell Physiol Biochem. 2018. PMID: 29680837
-
Serum-derived extracellular vesicles facilitate temozolomide resistance in glioblastoma through a HOTAIR-dependent mechanism.Cell Death Dis. 2022 Apr 13;13(4):344. doi: 10.1038/s41419-022-04699-8. Cell Death Dis. 2022. PMID: 35418162 Free PMC article.
Cited by
-
Prognostic role and functional impact of cadherin genes in non-small cell lung cancer tumorigenesis: mechanistic insights from in silico and in vitro analyses.PeerJ. 2025 Aug 19;13:e19785. doi: 10.7717/peerj.19785. eCollection 2025. PeerJ. 2025. PMID: 40860670 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

