Caplacizumab use in immune-mediated thrombotic thrombocytopenic purpura: an international multicentre retrospective Cohort study (The Capla 1000+ project)
- PMID: 40235949
- PMCID: PMC11997362
- DOI: 10.1016/j.eclinm.2025.103168
Caplacizumab use in immune-mediated thrombotic thrombocytopenic purpura: an international multicentre retrospective Cohort study (The Capla 1000+ project)
Abstract
Background: The anti-Von Willebrand Factor (VWF) nanobody caplacizumab is licensed for adults with immune-mediated thrombotic thrombocytopenic purpura (iTTP) in association with therapeutic plasma exchange (TPE) and immunosuppression. However, whether caplacizumab reduces mortality, and its optimal timing of initiation, is not completely settled.
Methods: This international, multicenter retrospective cohort study recruited patients from 2018 until 2023 and data collection took place from January 1st to June 30th 2023 in the participating centers. One thousand and fifteen patients were treated with daily TPE, immunosuppression with corticosteroids ± rituximab, and caplacizumab (caplacizumab group), which was compared to historic controls treated with TPE and corticosteroids ± rituximab (control group, N = 510). Caplacizumab initiation was classified as early (within 3 days; 76% of cases) or delayed (≥4 days from first TPE).
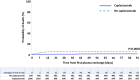
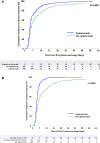
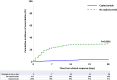
Findings: Three-month survival rate in the caplacizumab group was 98.5%, compared with 94% in controls (P < 0.0001). Three-month mortality rate was 4.2-fold higher in controls than in caplacizumab-treated patients (95% CI: 2.22-7.7, P < 0.0001), regardless of rituximab use. In both groups, death was observed primarily in elderly patients, and age was the factor most associated with 3-month mortality. Patients receiving caplacizumab showed reduced refractoriness, exacerbations, and required fewer TPE sessions to achieve clinical response versus controls (P < 0.0001 all). Time to clinical response in the caplacizumab group was shorter than in controls, and even shorter in patients with early caplacizumab initiation (P < 0.0001 both). Caplacizumab-related adverse events were observed in 21% of patients, with major bleeding in 2.4%, which was more common in elderly patients.
Interpretation: The early association of Caplacizumab to TPE and immunosuppression significantly reduces unfavorable outcomes during iTTP, including death, and alleviates the burden of care at the potential expense of bleeding events. Advanced age, however, remains an adverse factor for survival. The limitations of our study include its retrospective and multicentric design and the use of a historical control cohort.
Funding: None.
Keywords: ADAMTS13; Caplacizumab; Prognosis; Rituximab; Thrombotic thrombocytopenic purpura.
© 2025 The Author(s).
Conflict of interest statement
P. Coppo is member of the Clinical Advisory Board for Alexion, Sanofi-Genzyme and Takeda. L. Kühne received consulting fees from Alexion and research funding from Sanofi-Genzyme. L.A. Völker received research funding and consulting fees from Alexion, AstraZeneca, Bayer, GC Biopharm and Sanofi-Genzyme, GC Biopharm. P.T. Brinkkötter received speaker honoraria and consultant fees from AstraZeneca, Alexion, Bayer, Boehringer-Ingelheim, Novartis, Roche, Sanofi-Genzyme, Travere, Vifor CSL and participated in advisory boards for Alexion, Sanofi-Genzyme, Novartis, Travere, Takeda, Vifor CSL and Bayer. He declares research funding from the German Research Foundation BR-2955/8 and Sanofi-Genzyme. P. Knöbl is member of the Clinical Advisory Boards for Sanofi and Takeda. T. Boechat has participated to advisory boards for Sanofi-Genzyme. Y. Benhamou, B. Joly and A. Veyradier have participated to Advisory boards for Sanofi-Genzyme and Takeda. M.E. Mingot-Castellano is member of the Clinical Advisory Board for Alexion, Werfen, Sanofi-Genzyme and Takeda, and grants from the 3 companies. X. Long Zheng is a consultant for Alexion, Apollo, GC Biopharma, Sanofi-Genzyme, Stago, and Takeda. X.L.Z. is also the co-founder of Clotsolution; he also received educational grant funding to support 2024 ISTH congress satellite symposium for platelets and hemostasis, Bangkok, Thailand, and grants unrelated to the present study, from NHLBI (HL157975-01A1 and HL164016-01A1) and Answering T.T.P. Foundation. Marienn Reti received congress support by Sanofi-Genzyme (EHA, 2024) and participated to the iTTP European and International Region Medical Advisory Board (2023). C.J. Patriquin has receive consultancy honoraria from Alexion, BioCryst, Novartis, Roche, Sanofi-Genzyme, Sobi, and Takeda, and speaking honoraria from Alexion, Amgen, and Sobi. K. Pavenski provided unpaid consultancy to Sanofi-Genzyme and Takeda; served as site PI in industry trials of Sanofi-Genzyme, Takeda, Roche and SOBI. She received honoraria from France foundation for travel and presentations and honoraria from Octapharma for travel. K. Sakai received lecture fees from Sanofi-Genzyme. M. Matsumoto has provided consultancy services for Takeda, Alexion and Sanofi-Genzyme, and has received speaker fees for Takeda, Alexion, Asahikasei Pharma, and Sanofi-Genzyme and has received research funding from Alexion, Chugai Pharmaceutical, Asahikasei Pharma, and Sanofi-Genzyme. P. Agosti received honoraria for participating as a speaker at educational meetings organized by Sanofi-Genzyme. I. Mancini received honoraria for participating as a speaker at educational meetings organized by Werfen and Sanofi-Genzyme. F. Peyvandi received honoraria for participating in symposia and educational events for Takeda/Spark and in advisory boards for Biomarin, Roche, Sanofi-Genzyme, Sobi, and CSL Behring. S. Cataland is member of the Clinical Advisory Board for Sanofi-Genzyme and Takeda. M. Scully is member of the Clinical Advisory Board for Alexion, Sanofi-Genzyme and Takeda. B. Lämmle is chairman of data monitoring committees of studies investigating recombinant ADAMTS13 for the treatment of congenital and acquired TTP (Takeda), chairman of a steering committee analyzing global impact of congenital TTP (Takeda), and chairman of the data monitoring committee of Mayari study (investigating caplacizumab for the treatment of autoimmune TTP without plasma exchange (Sanofi-Genzyme)). The other authors do not have any conflict of interest to declare.
Figures

References
-
- Kremer Hovinga J.A., Coppo P., Lämmle B., Moake J.L., Miyata T., Vanhoorelbeke K. Thrombotic thrombocytopenic purpura. Nat Rev Dis Primer. 2017;3 - PubMed
-
- Rock G.A., Shumak K.H., Buskard N.A., et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis Study Group. N Engl J Med. 1991;325:393–397. - PubMed
-
- Kremer Hovinga J.A., Vesely S.K., Terrell D.R., Lämmle B., George J.N. Survival and relapse in patients with thrombotic thrombocytopenic purpura. Blood. 2010;115:1500–1511. - PubMed
-
- George J.N. Corticosteroids and rituximab as adjunctive treatments for thrombotic thrombocytopenic purpura. Am J Hematol. 2012;87(Suppl 1):S88–S91. - PubMed
-
- Scully M., McDonald V., Cavenagh J., et al. A phase 2 study of the safety and efficacy of rituximab with plasma exchange in acute acquired thrombotic thrombocytopenic purpura. Blood. 2011;118:1746–1753. - PubMed

