This is a preprint.
Structural Insights into Kainate Receptor Desensitization
- PMID: 40236080
- PMCID: PMC11996427
- DOI: 10.1101/2025.03.27.645769
Structural Insights into Kainate Receptor Desensitization
Update in
-
Structural insights into kainate receptor desensitization.Nat Commun. 2025 Dec 9;16(1):10989. doi: 10.1038/s41467-025-65920-8. Nat Commun. 2025. PMID: 41365859 Free PMC article.
Abstract
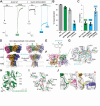
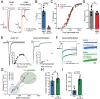
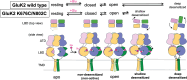
Kainate receptors (KARs), along with AMPA and NMDA receptors, belong to the ionotropic glutamate receptor (iGluR) family and play critical roles in mediating excitatory neurotransmission throughout the central nervous system. KARs also regulate neurotransmitter release and modulate neuronal excitability and plasticity. Receptor desensitization plays a critical role in modulating the strength of synaptic transmission and synaptic plasticity. While KARs share overall structural similarity with AMPA receptors, the desensitized state of KARs differs strikingly from that of other iGluRs. Despite extensive studies on KARs, a fundamental question remains unsolved: why do KARs require large conformational changes upon desensitization, unlike other iGluRs? To address this, we present cryo-electron microscopy structures of GluK2 with double cysteine mutations in non-desensitized, shallow-desensitized and deep-desensitized conformations. In the shallow-desensitized conformation, two cysteine crosslinks stabilize the receptors in a conformation that resembles the desensitized state of AMPA receptors. However, unlike the tightly closed pore observed in the deep-desensitized KAR and desensitized AMPAR conformations, the channel pore in the shallow-desensitized state remains incompletely closed. Patch-clamp recordings and fluctuation analysis suggest that this state remains ion-permeable, indicating that the lateral rotational movement of KAR ligand-binding domains (LBDs) is critical for complete channel closure and stabilization of the receptor in desensitization states. Together with the multiple conformations representing different degree of desensitization, our results define the unique mechanism and conformational dynamics of KAR desensitization.
Keywords: Kainate-type ionotropic glutamate receptors; desensitization mechanism; structure.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures





References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
