Factor XI localization in human deep venous thrombus and function of activated factor XI on venous thrombus formation and hemostasis
- PMID: 40236284
- PMCID: PMC11999338
- DOI: 10.1016/j.rpth.2025.102720
Factor XI localization in human deep venous thrombus and function of activated factor XI on venous thrombus formation and hemostasis
Abstract
Background: Novel anticoagulants targeting coagulation factor (F)XI/activated FXI (FXIa) are currently under development. However, whether FXI is present in human deep vein thrombosis (DVT) and whether FXIa and activated FX (FXa) play different roles in venous thrombus formation and hemostasis remain unclear.
Objectives: To determine the presence of FXI in DVT and the effects of direct oral FXIa and FXa inhibitors on venous thrombus formation and hemostasis in rabbits and on in vitro thrombus formation.
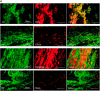
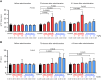
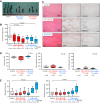
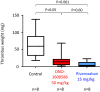
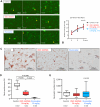
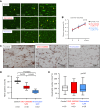
Methods: We immunohistochemically assessed FXI localization in human-aspirated DVT (n = 15). Additionally, we compared thrombus formation induced by endothelial denudation and stenosis or stasis in the jugular vein and skin bleeding time and volume between rabbits treated with direct FXIa inhibitors (ONO-1600586) and FXa inhibitors (rivaroxaban). Ex vivo rabbit and human blood were perfused in a flow chamber under low-shear rates (70/s).
Results: FXI was localized in all DVT, predominantly in fibrin-rich areas. The FXI immunopositive area in the nonorganizing area was greater than that in the organizing area. Although FXIa and FXa inhibitors comparably inhibited venous thrombus formation, FXIa inhibitors did not affect bleeding time or volume in rabbits. FXIa or FXa inhibitors mildly or strongly inhibited fibrin formation at low-shear rates, respectively. Furthermore, the FXIa inhibitor suppressed human FXIa activity, thrombin generation, and fibrin formation during perfusion.
Conclusion: The pathologic findings of human DVT suggest FXI's role in human DVT. FXIa inhibitors may inhibit less fibrin formation than FXa inhibitors and may explain the minor role of FXIa in hemostasis.
Keywords: factor X; factor XI; hemostasis; pathology; venous thromboembolism.
© 2025 The Author(s).
Figures




References
-
- Wendelboe A.M., Raskob G.E. Global burden of thrombosis: epidemiologic aspects. Circ Res. 2016;118:1340–1347. - PubMed
-
- Carnicelli A.P., Hong H., Connolly S.J., Eikelboom J., Giugliano R.P., Morrow D.A., et al. Direct oral anticoagulants versus warfarin in patients with atrial fibrillation: patient-level network meta-analyses of randomized clinical trials with interaction testing by age and sex. Circulation. 2022;145:242–255. - PMC - PubMed
-
- Gailani D., Broze G.J., Jr. Factor XI activation in a revised model of blood coagulation. Science. 1991;253:909–912. - PubMed
-
- Meijers J.C., Tekelenburg W.L., Bouma B.N., Bertina R.M., Rosendaal F.R. High levels of coagulation factor XI as a risk factor for venous thrombosis. N Engl J Med. 2000;342:696–701. - PubMed
LinkOut - more resources
Full Text Sources

