Impact of Shipping Transit Time on Central Laboratory Processing of Total Colony Forming Units (CFU) and Staphylococcus aureus Detection
- PMID: 40236356
- PMCID: PMC11998623
- DOI: 10.7759/cureus.80590
Impact of Shipping Transit Time on Central Laboratory Processing of Total Colony Forming Units (CFU) and Staphylococcus aureus Detection
Abstract
Background: Central laboratory processing of anesthesia work area reservoir samples is used to improve infection control measures. Reservoir samples returning ≥ 100 colony forming units (CFU) and Staphylococcus aureus (S. aureus) detection are monitored to identify improvement targets. The impact of sample shipment time under ambient conditions on these meaningful outcomes has not been characterized. Such insight could help to further optimize feedback that has been proven to generate substantial reductions in surgical site infections. In this study, we aimed to assess the impact of ambient shipping conditions on patient intravenous stopcock sample CFU ≥ 100 and S. aureus detection because stopcock contamination is repeatedly associated with increased patient mortality.
Methods: We conducted a retrospective analysis involving seven geographically dispersed hospitals over a 4.2-year (October 1, 2018 to December 31, 2022) study period. We chose geographically dispersed sites considering variation in ambient shipping conditions and time. Stopcocks sampled at the end of surgery were shipped to a central laboratory, plated to sheep's blood agar, incubated for 24hr at 36°C, CFU/mL quantified, and distinct isolates assessed by colony morphology, Gram stain, simple rapid tests (e.g., coagulase, oxidase, lactose fermentation, catalase), and selective growth medium.
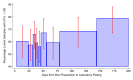
Results: A total of 969 stopcock samples were analyzed. The percentage of stopcocks with CFU ≥ 100 was stable following sample collection from days 3 to 32 (odds ratio (OR) 1.0086/day, 95% confidence interval (CI) 0.9868-1.0309/day) and from sample kit preparation from days 3 to 143 (OR 1.0044/day, 95% CI 0.9991-1.0099/day). S. aureus detection decreased beyond 14 days from the period of collection during the surgical procedure (P = 0.0024; OR 0.83, 95% CI 0.21-0.71).
Conclusions: When utilizing a central laboratory for processing anesthesia workspace reservoir stopcock set samples, there is stability of ≥ 100 CFU for up to 32 days from collection and up to 143 days from kit preparation. S. aureus detection remains stable for up to 14 days. Therefore, when monitoring stopcock contamination to provide feedback, samples should be processed within 14 days from their collection. Anticipated shipment times should be considered by sample collection personnel to ensure optimal sample yield.
Keywords: central clinical laboratory; colony forming units (cfu); detection; s aureus; shipment stability; transit time.
Copyright © 2025, Loftus et al.
Conflict of interest statement
Human subjects: All authors have confirmed that this study did not involve human participants or tissue. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: Randy W. Loftus declare(s) a patent and stock/stock options from RDB Bioinformatics. Patent pending. Randy W. Loftus and Jeremiah R. Brown declare(s) a grant from National Institutes of Health. R01AI155752. Franklin Dexter declare(s) Franklin Dexter declare(s) that the Division of Management Consulting of the University of Iowa’s Department of Anesthesia provides consultations to corporations, hospitals, and individuals. Dr. Dexter receives no funds personally other than his salary and allowable expense reimbursements from the University of Iowa and has tenure with no incentive program. He and his family have no financial holdings in any company related to his work, other than indirectly through mutual funds for retirement. Income from the Division's consulting work is used to fund Division research. A list of all the Division’s consults is available in his posted curriculum vitae at https://FranklinDexter.net/Contact_Info.htm from University of Iowa. Intellectual property info: Multi-level, Laboratory-Based Surveillance System for Detection of Intraoperative “Eskape” Bacterial Pathogens for HCAI Prevention (Publication number: 20190226004, publication date: July 25, 2019, inventor: Randy W. Loftus). Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures


Similar articles
-
A threshold of 100 or more colony-forming units on the anesthesia machine predicts bacterial pathogen detection: a retrospective laboratory-based analysis.Can J Anaesth. 2024 May;71(5):600-610. doi: 10.1007/s12630-024-02707-3. Epub 2024 Feb 27. Can J Anaesth. 2024. PMID: 38413516 English.
-
Erratum: High-Throughput Identification of Resistance to Pseudomonas syringae pv. Tomato in Tomato using Seedling Flood Assay.J Vis Exp. 2023 Oct 18;(200). doi: 10.3791/6576. J Vis Exp. 2023. PMID: 37851522
-
Leaving more than your fingerprint on the intravenous line: a prospective study on propofol anesthesia and implications of stopcock contamination.Anesth Analg. 2015 Apr;120(4):861-7. doi: 10.1213/ANE.0b013e318292ed45. Anesth Analg. 2015. PMID: 23749446 Free PMC article.
-
Interventions to reduce contaminated aerosols produced during dental procedures for preventing infectious diseases.Cochrane Database Syst Rev. 2020 Oct 12;10(10):CD013686. doi: 10.1002/14651858.CD013686.pub2. Cochrane Database Syst Rev. 2020. PMID: 33047816 Free PMC article.
-
Lessons from the organization of a proficiency testing program in food microbiology by interlaboratory comparison: analytical methods in use, impact of methods on bacterial counts and measurement uncertainty of bacterial counts.Food Microbiol. 2006 Feb;23(1):1-38. doi: 10.1016/j.fm.2005.01.010. Food Microbiol. 2006. PMID: 16942983 Review.
Cited by
-
The Importance of the Detection of Staphylococcus aureus Strain Characteristics Associated With Perioperative Transmission of Antibiotic Resistance.Cureus. 2025 Apr 8;17(4):e81885. doi: 10.7759/cureus.81885. eCollection 2025 Apr. Cureus. 2025. PMID: 40342447 Free PMC article.
References
-
- Effectiveness and feasibility of an evidence-based intraoperative infection control program targeting improved basic measures: a post-implementation prospective case-cohort study. Wall RT, Datta S, Dexter F, et al. J Clin Anesth. 2022;77:110632. - PubMed
-
- Operating room PathTrac analysis of current intraoperative Staphylococcus aureus transmission dynamics. Robinson AD, Dexter F, Renkor V, Reddy S, Loftus RW. Am J Infect Control. 2019;47:1240–1247. - PubMed
-
- High-risk Staphylococcus aureus transmission in the operating room: a call for widespread improvements in perioperative hand hygiene and patient decolonization practices. Loftus RW, Dexter F, Robinson AD. Am J Infect Control. 2018;46:1134–1141. - PubMed
-
- Multiple reservoirs contribute to intraoperative bacterial transmission. Loftus RW, Brown JR, Koff MD, et al. Anesth Analg. 2012;114:1236–1248. - PubMed
LinkOut - more resources
Full Text Sources
