Analysis of treatment efficacy, tolerability, and survival of patients receiving antifibrotic therapy for progressive nonidiopathic pulmonary fibrosis
- PMID: 40236382
- PMCID: PMC11996130
- DOI: 10.4103/atm.atm_213_24
Analysis of treatment efficacy, tolerability, and survival of patients receiving antifibrotic therapy for progressive nonidiopathic pulmonary fibrosis
Abstract
Background: There are still disagreements about diagnostic criteria and treatment of progressive pulmonary fibrosis (PPF). Real-life data and survival analyses have a guiding role in clarifying this issue.
Methods: In this multicenter retrospective cohort study, real-life data of adult patients diagnosed with PPF and treated with antifibrotics for at least 6 months were examined.
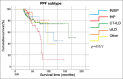
Results: Of the 222 patients, 161 were treated with Nintedanib (N) and 61 with Pirfenidone (P). The most common PPF subtype was connective tissue disease-related interstitial lung disease (CTD-ILD) (53.2%). The progression rate was significantly higher in patients with usual interstitial pneumonia (UIP) (P = 0.003). A -3.1% (-49.2 ml) decrease was detected in forced vital capacity (FVC) in the 6th month. The 6th month and overall progression-free survival (PFS) rates were 83.3% and 51.8%. The 6th month and overall clinical event-free survival (CEFS) rates were 89.6% and 53.6%. The survival rates for 6th, 12th, and entire follow-up periods were found to be 98.2%, 89.2%, and 77.5%. CT-ILD had the longest survival time (166.5 ± 9.2 months) and fibrotic hypersensitivity pneumonia had the shortest survival time (87.6 ± 9.2 months) (P = 0.011). N was advantageous in patients with UIP in terms of FVC loss and estimated survival. While PFS during the entire follow-up period was in favor of N, CEFS had no significant difference between drugs.
Conclusion: PPF subtypes have significant differences in terms of prognosis and survival. The effect of AF drugs on progression varies, especially among radiological patterns. An individualized approach is required in the diagnosis, follow-up, and treatment of patients with PPF.
Keywords: Antifibrotic; idiopathic pulmonary fibrosis; nintedanib; pirfenidone; pulmonary fibrosis; survival.
Copyright: © 2025 Annals of Thoracic Medicine.
Conflict of interest statement
There are no conflicts of interest.
Figures


References
-
- Cilli A, Ocal N, Uzer F, Coskun F, Sevinc C, Ursavas A, et al. Elderly idiopathic pulmonary fibrosis patients remain on therapy despite higher incidence of adverse events and dose reductions. Respir Investig. 2023;61:490–7. - PubMed
-
- Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh SL, Inoue Y, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med. 2019;381:1718–27. - PubMed
-
- Behr J, Prasse A, Kreuter M, Johow J, Rabe KF, Bonella F, et al. Pirfenidone in patients with progressive fibrotic interstitial lung diseases other than idiopathic pulmonary fibrosis (RELIEF): A double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir Med. 2021;9:476–86. - PubMed
-
- Distler O, Highland KB, Gahlemann M, Azuma A, Fischer A, Mayes MD, et al. Nintedanib for systemic sclerosis-associated interstitial lung disease. N Engl J Med. 2019;380:2518–28. - PubMed
LinkOut - more resources
Full Text Sources
