This is a preprint.
Integrating Pulmonary and Systemic Transcriptomic Profiles to Characterize Lung Injury after Pediatric Hematopoietic Stem Cell Transplant
- PMID: 40236411
- PMCID: PMC11998824
- DOI: 10.1101/2025.03.31.25324969
Integrating Pulmonary and Systemic Transcriptomic Profiles to Characterize Lung Injury after Pediatric Hematopoietic Stem Cell Transplant
Update in
-
Integrating pulmonary and systemic transcriptomes to characterize lung injury after pediatric hematopoietic stem cell transplant.JCI Insight. 2025 Jul 22;10(17):e194440. doi: 10.1172/jci.insight.194440. eCollection 2025 Sep 9. JCI Insight. 2025. PMID: 40694427 Free PMC article.
Abstract
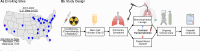
Hematopoietic stem cell transplantation (HCT) is potentially curative for numerous malignant and non-malignant diseases but can lead to lung injury due to chemoradiation toxicity, infection, and immune dysregulation. Bronchoalveolar lavage (BAL) is the most commonly used procedure for diagnostic sampling of the lung but is invasive, cannot be performed in medically fragile patients, and is challenging to perform serially. We previously showed that BAL transcriptomes representing pulmonary inflammation and cellular injury can phenotype post-HCT lung injury and predict mortality outcomes. However, whether peripheral blood testing is a suitable minimally-invasive surrogate for pulmonary sampling in the HCT population remains unknown. To address this question, we compared 210 paired BAL and peripheral blood transcriptomes obtained from 166 pediatric HCT patients at 27 children's hospitals. BAL and blood mRNA abundance showed minimal overall correlation at the level of individual genes, gene set enrichment scores, imputed cell fractions, and T- and B-cell receptor clonotypes. Instead, we identified significant site-specific transcriptional programs. In BAL, expression of innate and adaptive immune pathways was tightly co-regulated with expression of epithelial mesenchymal transition and hypoxia pathways, and these signatures were associated with mortality. In contrast, in blood, expression of endothelial injury, DNA repair, and cellular metabolism pathways was associated with mortality. Integration of paired BAL and blood transcriptomes dichotomized patients into two groups, of which one group showed twice the rate of hypoxia and significantly worse outcomes within 7 days of enrollment. These findings reveal a compartmentalized injury response, where BAL and peripheral blood transcriptomes provide distinct but complementary insights into local and systemic mechanisms of post-HCT lung injury.
Conflict of interest statement
COMPETING INTERESTS: M.S.Z. discloses consulting and advisory board work (Roche). C.C.D. discloses consulting and advisory board work (Jazz Pharmaceuticals; Alexion Inc.). J.J.A. discloses consulting and advisory board work (AscellaHealth; Takeda). T.C.Q. discloses speaker bureau, consulting and advisory board work (Alexion AstraZeneca Rare Disease; Jazz Pharmaceuticals). H.A-A. discloses research support (Adaptive). R.P. discloses consulting and advisory board work (BlueBird Bio) and research support (Amgen). M.A.P. discloses consulting and advisory board work (Novartis; Garuda; Autolous; Pfizer; Cargo; BlueBird Bio; Vertex) and research support (Miltenyi; Adaptive). L.N.S. discloses consulting and advisory board work (Sanofi). J.J.B. discloses consulting and advisory board work (Sanofi; BlueRock; Sobi; SmartImmune; Immusoft; Advanced Clinical; Merck). J.L.D. discloses salary support and research support (Chan Zuckerberg Biohub).
Figures




References
-
- Panoskaltsis-Mortari A, Griese M, Madtes DK, et al. An official American Thoracic Society research statement: noninfectious lung injury after hematopoietic stem cell transplantation: idiopathic pneumonia syndrome. Am J Respir Crit Care Med. 2011;183(9):1262–1279. doi: 10.1164/rccm.2007-413ST - DOI - PMC - PubMed
-
- Zinter MS, Holubkov R, Steurer MA, et al. Pediatric Hematopoietic Cell Transplant Patients Who Survive Critical Illness Frequently Have Significant but Recoverable Decline in Functional Status. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2018;24(2):330–336. doi: 10.1016/j.bbmt.2017.10.036 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
