This is a preprint.
Development and validation of a harmonized memory score for multicenter Alzheimer's disease and related dementia research
- PMID: 40236433
- PMCID: PMC11998833
- DOI: 10.1101/2025.03.31.25324964
Development and validation of a harmonized memory score for multicenter Alzheimer's disease and related dementia research
Abstract
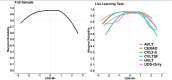
Introduction: List-learning tasks are important for characterizing memory in ADRD research, but the Uniform Data Set neuropsychological battery (UDS-NB) lacks a list-learning paradigm; thus, sites administer a range of tests. We developed a harmonized memory composite that incorporates UDS memory tests and multiple list-learning tasks.
Methods: Item-banking confirmatory factor analysis was applied to develop a memory composite in a diagnostically heterogenous sample (n=5943) who completed the UDS-NB and one of five list-learning tasks. Construct validity was evaluated through associations with demographics, disease severity, cognitive tasks, brain volume, and plasma phosphorylated tau (p-tau181 and p-tau217). Test-retest reliability was assessed. Analyses were replicated in a racially/ethnically diverse cohort (n=1058).
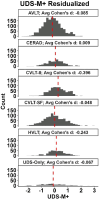
Results: Fit indices, loadings, distributions, and test-retest reliability were adequate. Expected associations with demographics and clinical measures within development and validation cohorts supported validity.
Discussion: This composite enables researchers to incorporate multiple list-learning tasks with other UDS measures to create a single metric.
Keywords: Alzheimer’s Dementia and Related Disorders; Co-calibration; Cognition; Harmonization; Memory; Neurodegeneration; Neuropsychology.
Conflict of interest statement
Conflicts of interest B.F.B. has served as an investigator for clinical trials sponsored by Alector, Biogen, Transposon and Cognition Therapeutics. He serves on the Scientific Advisory Board of the Tau Consortium, which is funded by the Rainwater Charitable Foundation. He receives research support from NIH. A.L.B. receives research support from the NIH, the Tau Research Consortium, the Association for Frontotemporal Degeneration, Bluefield Project to Cure Frontotemporal Dementia, Corticobasal Degeneration Solutions, the Alzheimer’s Drug Discovery Foundation and the Alzheimer’s Association. He has served as a consultant for Aeovian, AGTC, Alector, Arkuda, Arvinas, Boehringer Ingelheim, Denali, GSK, Life Edit, Humana, Oligomerix, Oscotec, Roche, TrueBinding and Wave and received research support from Biogen, Eisai and Regeneron. M.M.G. reports personal stock in Abbvie. R.C.P. reports personal fees from Roche, no personal fees from Eisai, and personal fees from Genentech, personal fees from Eli Lilly and personal fees from Nestle, outside the submitted work. B.L.M reported serving on the scientific advisory board of the Bluefield Project to Cure Frontotemporal Dementia; the John Douglas French Alzheimer’s Foundation; Fundación Centro de Investigación Enfermedades Neurológicas, Madrid, Spain; Genworth; the Kissick Family Foundation; the Larry L. Hillblom Foundation; and the Tau Consortium of the Rainwater Charitable Foundation; serving as a scientific advisor for the Arizona Alzheimer’s Consortium; Massachusetts General Hospital Alzheimer’s Disease Research Center; and the Stanford University Alzheimer’s Disease Research Center; receiving royalties from Cambridge University Press, Elsevier, Guilford Publications, Johns Hopkins Press, Oxford University Press, and the Taylor & Francis Group; serving as editor for Neurocase and section editor for Frontiers in Neurology; and receiving grants for the University of California San Francisco Frontotemporal Dementia Core, from the Bluefield Project to Cure Frontotemporal Dementia, and from the National Institute on Aging for the US–South American Initiative for Genetic-Neural-Behavioral Interactions in Human Neurodegenerative Diseases. G.D.R. reported grants from National Institutes of Health during the conduct of the study; consulting fees from C2N, Eli Lilly, Alector, Merck, Roche, and Novo Nordisk; data safety monitoring board fees from Johnson & Johnson; and grants from Avid Radiopharmaceuticals, GE Healthcare, Life Molecular Imaging, and Genentech outside the submitted work; and served as Associate Editor at JAMA Neurology. A.M.S. reported grants from the National Institutes of Health, the Bluefield Project to Cure Frontotemporal Dementia, and the Association for Frontotemporal Degeneration; personal fees from Alector, Prevail Therapuetics/Eli Lilly, Passage Bio, Takeda, and the Alzheimer’s Drug Discovery Foundation; and other from Datacubed Health (licensing fees) outside the submitted work. H. J. R. reported consulting fees from Genentech and Eisai outside the submitted work. Dr Staffaroni reported grants from the National Institutes of Health, the Bluefield Project to Cure Frontotemporal Dementia, and the Association for Frontotemporal Degeneration; personal fees from Alector, Prevail Therapuetics/Eli Lilly, Passage Bio, Takeda, and the Alzheimer’s Drug Discovery Foundation; and other from Datacubed Health (licensing fees) outside the submitted work. C.D. serves as a consultant to Norvo Nordisk and Eisai Pharmaceuticals. D.K.J. has stock holdings in Sage Cerebrovascular Diagnostics, serves as President for Sage Cerebrovascular Diagnostics, and has a patent pending for Serologic assay for silent brain ischemia licensed to Sage Cerebrovascular Diagnostics
Figures



References
-
- Rabin L.A., Paolillo E., and Barr W.B., Stability in test-usage practices of clinical neuropsychologists in the United States and Canada over a 10-year period: A follow-up survey of INS and NAN members. Archives of Clinical Neuropsychology, 2016. 31(3): p. 206–230. - PubMed
-
- Mansbach W.E., Mace R.A., and Clark K.M., Story recall and word lists: Differential and combined utilities in predicting cognitive diagnosis. Journal of Clinical and Experimental Neuropsychology, 2014. 36(6): p. 569–576. - PubMed
-
- De Simone M.S., et al. , Different deficit patterns on word lists and short stories predict conversion to Alzheimer’s disease in patients with amnestic mild cognitive impairment. Journal of Neurology, 2017. 264: p. 2258–2267. - PubMed
-
- Tremont G., et al. , Comparison of verbal memory impairment rates in mild cognitive impairment. Journal of Clinical and Experimental Neuropsychology, 2010. 32(6): p. 630–636. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
