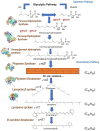
Biosynthetic Pathway of psi, psi-Carotene from Streptomyces sp. VITGV38 (MCC 4869)
- PMID: 40236476
- PMCID: PMC11998277
- DOI: 10.3389/fmicb.2025.1548894
Biosynthetic Pathway of psi, psi-Carotene from Streptomyces sp. VITGV38 (MCC 4869)
Abstract
Introduction: Endophytic Streptomyces play a crucial role in plant-microbe interactions, often exhibiting beneficial biological activities, including the production of bioactive secondary metabolites. This study aimed to characterize the carotene biosynthetic pathway of a newly discovered Streptomyces sp. VITGV38, isolated from tomato (Lycopersicon esculentum).
Methods: The strain (Streptomyces sp. VITGV38, MCC4869) was cultured in starch casein broth, and its metabolite profile was analyzed using Gas Chromatography-Mass Spectrometry (GC-MS). Whole-genome sequencing was performed using the Illumina platform, and the biosynthetic gene clusters (BGCs) were identified using antiSMASH.
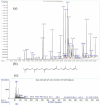
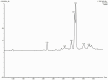
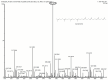
Results: Metabolite analysis revealed the presence of pigmented compounds, including psi, psi-carotene, detected at a retention time of 25.094, constituting 1.26% of the crude extract. Whole-genome sequencing uncovered an 8.27 Mb genome encoding 26 distinct secondary metabolite biosynthetic gene clusters. Notably, scaffold 26.3 was identified as a terpene biosynthetic cluster, accounting for 62% of the total secondary metabolite content and associated with carotenoid and β-carotene production.
Discussion: These findings highlight the biotechnological potential of Streptomyces sp. VITGV38 for sustainable microbial production of carotenoids, offering an eco-friendly alternative to synthetic pigments. This study provides valuable insights into microbial carotenoid biosynthesis and its potential industrial applications.
Keywords: Streptomyces sp. VITGV38; antiSMASH; biosynthetic gene cluster; pigment; psi; psi-carotene; secondary metabolite.
Copyright © 2025 Pattapulavar, Ramanujam, Sekaran, Chandrasekaran, Panchal and Christopher.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Al-Dhabi N. A., Esmail G. A., Ghilan A. K. M., Arasu M. V., Duraipandiyan V., Ponmurugan K. (2020). Chemical constituents of Streptomyces sp. strain Al-Dhabi-97 isolated from the marine region of Saudi Arabia with antibacterial and anticancer properties. J. Infect. Public Health 13, 235–243. doi: 10.1016/J.JIPH.2019.09.004, PMID: - DOI - PubMed
-
- Becerril A., Álvarez S., Braña A. F., Rico S., Díaz M., Santamaría R. I., et al. (2018). Uncovering production of specialized metabolites by Streptomyces argillaceus: activation of cryptic biosynthesis gene clusters using nutritional and genetic approaches. PLoS One 13:e0198145. doi: 10.1371/JOURNAL.PONE.0198145, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

