Virally mediated enhancement of efferent inhibition reduces acoustic trauma in wild-type murine cochleas
- PMID: 40236498
- PMCID: PMC11999434
- DOI: 10.1016/j.omtm.2025.101455
Virally mediated enhancement of efferent inhibition reduces acoustic trauma in wild-type murine cochleas
Abstract
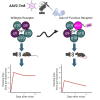
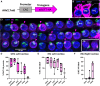
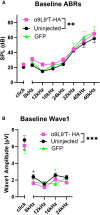
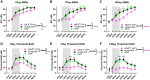
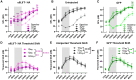
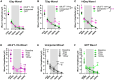
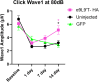
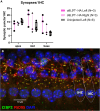
Noise-induced hearing loss (NIHL) poses an emerging global health problem with only ear protection or sound avoidance as preventive strategies. The cochlea receives some protection from medial olivocochlear efferent neurons, providing a potential target for therapeutic enhancement. Cholinergic efferents release acetylcholine (ACh) to hyperpolarize and shunt the outer hair cells (OHCs), reducing sound-evoked activation. The (α9)2(α10)3 nicotinic ACh receptor (nAChR) on the OHCs mediates this effect. Transgenic knockin mice with a gain-of-function nAChR (α9L9'T) suffer less NIHL. α9 knockout mice are more vulnerable to NIHL but can be rescued by viral transduction of the α9L9'T subunit. In this study, an HA-tagged gain-of-function α9 isoform was expressed in wild-type mice to reduce NIHL. Synaptic integration of the virally expressed nAChR subunit was confirmed by HA immunopuncta localized to the postsynaptic membrane of OHCs. After noise exposure, AAV2.7m8-CAG-α9L9'T-HA (α9L9'T-HA)-injected mice had less hearing loss (auditory brainstem response [ABR] thresholds and threshold shifts) than did control mice. ABRs of α9L9'T-HA-injected mice also had larger wave-1 amplitudes and better recovery of wave-1 amplitudes post noise exposure. Thus, virally expressed α9L9'T combines effectively with native α9 and α10 subunits to mitigate NIHL in wild-type cochleas.
Keywords: adeno-associated virus; cholinergic inhibition; cochlear efferents; gene therapy; nicotinic receptor; noise-induced hearing loss; olivocochlear system; outer hair cell; posterior semicircular canal injection.
© 2025 The Authors.
Conflict of interest statement
US and International Patent Application US2023/033849, filed 9/27/2023, “Cholinergic Gene Therapy to Preserve Hearing” (P.A.F.).
Figures
Update of
-
Virally-Mediated Enhancement of Efferent Inhibition Reduces Acoustic Trauma in Wild Type Murine Cochleas.bioRxiv [Preprint]. 2024 Sep 15:2024.09.12.612688. doi: 10.1101/2024.09.12.612688. bioRxiv. 2024. Update in: Mol Ther Methods Clin Dev. 2025 Mar 21;33(2):101455. doi: 10.1016/j.omtm.2025.101455. PMID: 39314296 Free PMC article. Updated. Preprint.
References
Grants and funding
LinkOut - more resources
Full Text Sources

