An Injectable Ibuprofen Sustained-Release Composite Hydrogel System Effectively Accelerates Diabetic Wound Healing via Anti-Inflammatory Effects and Angiogenesis
- PMID: 40236520
- PMCID: PMC11998958
- DOI: 10.2147/IJN.S504924
An Injectable Ibuprofen Sustained-Release Composite Hydrogel System Effectively Accelerates Diabetic Wound Healing via Anti-Inflammatory Effects and Angiogenesis
Abstract
Purpose: Excessive inflammation in diabetic wounds, driven by hyperglycemia, prolongs healing, increases the risk of non-healing ulcers, and can lead to severe complications such as amputation or life-threatening infections. Recurrent wound infections and prolonged treatment impose significant economic and psychological burdens, drastically reducing patients' quality of life. Modulating the inflammatory response is a promising strategy to accelerate diabetic wound healing. Ibuprofen (IBU), a widely used anti-inflammatory and analgesic agent, has the potential to promote healing by mitigating excessive inflammation and alleviating wound-associated pain. However, its clinical application is hindered by poor water solubility and a short half-life. Therefore, a controlled and sustained-release system for IBU could enhance its therapeutic efficacy in diabetic wound management.
Materials and methods: Here, we present an in situ multi-crosslinked composite hydrogel system that integrates oxidized alginate (OSA), methacryloylated gelatin (GelMA), and an ibuprofen/amino-modified β-cyclodextrin inclusion complex (IBU/CD-NH2) via ion crosslinking, photocrosslinking, and Schiff-base reactions.
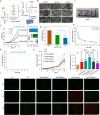
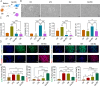
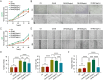
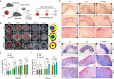
Results: The optimized hydrogel formulation was synthesized at 35°C, with a P/A molar ratio of 2 and an methacrylamide(MA) volume fraction of 20%. Physicochemical and biocompatibility analyses demonstrated that the IBU-loaded composite hydrogel exhibits enhanced mechanical strength, favorable biocompatibility, tunable degradation, and injectability. This system effectively addresses IBU's solubility and absorption challenges while conforming to wounds of varying shapes and sizes, enabling controlled and sustained drug release. Cellular and animal studies confirmed that the hydrogel continuously and uniformly releases IBU, exerting anti-inflammatory effects while promoting angiogenesis and fibroblast migration. This leads to enhanced granulation tissue formation, collagen deposition, and epidermal regeneration, significantly accelerating wound closure within 14 days.
Conclusion: By simultaneously suppressing inflammation and stimulating tissue regeneration through controlled IBU release, this hydrogel system offers a highly effective strategy for diabetic wound healing and holds strong potential for clinical application.
Keywords: diabetic wound healing; ibuprofen; inflammation; injectable composite hydrogel system; sustained-release.
© 2025 Li et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


Similar articles
-
Injectable Nanocomposite Hydrogel for Accelerating Diabetic Wound Healing Through Inflammatory Microenvironment Regulation.Int J Nanomedicine. 2025 Feb 6;20:1679-1696. doi: 10.2147/IJN.S505918. eCollection 2025. Int J Nanomedicine. 2025. PMID: 39931526 Free PMC article.
-
Oxidized regenerated cellulose-based supermolecular hydrogel for controlled drug release: Integrating cyclodextrin-mediated hydrophobic drug distribution, wound-specific degradation, and multifunctional therapeutic effects.Int J Biol Macromol. 2025 May;310(Pt 3):143445. doi: 10.1016/j.ijbiomac.2025.143445. Epub 2025 Apr 22. Int J Biol Macromol. 2025. PMID: 40274142
-
Intelligent sequential degradation hydrogels by releasing bimetal-phenolic for enhanced diabetic wound healing.J Control Release. 2025 Feb 10;378:961-981. doi: 10.1016/j.jconrel.2024.12.055. Epub 2025 Jan 1. J Control Release. 2025. PMID: 39724946
-
A Comprehensive Review of Honey-Containing Hydrogel for Wound Healing Applications.Gels. 2025 Mar 12;11(3):194. doi: 10.3390/gels11030194. Gels. 2025. PMID: 40136899 Free PMC article. Review.
-
Diabetic Wound Healing: Factors, Mechanisms, and Treatment Strategies Using Herbal Components.Curr Drug Targets. 2025;26(6):367-381. doi: 10.2174/0113894501354898241220075327. Curr Drug Targets. 2025. PMID: 39791148 Review.
Cited by
-
Exo-hydrogel therapy: a revolutionary approach to managing diabetic complications.J Nanobiotechnology. 2025 Aug 11;23(1):558. doi: 10.1186/s12951-025-03621-6. J Nanobiotechnology. 2025. PMID: 40790200 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

