Vegetal oil-based ketogenic diet improves inflammation and fibrosis in experimental metabolic dysfunction-associated steatohepatitis
- PMID: 40236713
- PMCID: PMC11996634
- DOI: 10.3389/fimmu.2025.1518687
Vegetal oil-based ketogenic diet improves inflammation and fibrosis in experimental metabolic dysfunction-associated steatohepatitis
Abstract
Background and aims: Metabolic dysfunction-associated steatohepatitis (MASH) represents a growing cause of liver cirrhosis and hepatocellular carcinoma (HCC). However, effective therapy for MASH is still lacking. Despite recent studies suggest that ketosis might improve MASH evolution, the mechanisms involved have not been explored since common ketogenic diets cause severe steatohepatitis in mice. In this study, we have investigated the capacity of a new-formulated ketogenic diet (KD) containing vegetal fat in improving liver alterations associated with experimental MASH.
Methods: MASH was induced in C57BL/6 mice by feeding a cholesterol-enriched Western Diet (WD) for up to 16 weeks, followed by switching animals to KD for an additional eight weeks.
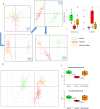
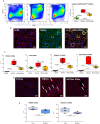
Results: We observed that KD administration greatly increased ketone body production and significantly reduced liver and body weights. Moreover, liver proteomic analysis and functional tests evidenced an improved glucose and lipid metabolism along with insulin resistance in KD-fed mice. These metabolic effects were associated with an amelioration in MASH-associated gut dysbiosis and with an improvement of hepatic steatosis, parenchymal injury and liver fibrosis. From the mechanistic point of view mice receiving KD showed a significant reduction in liver TREM2-positive monocyte-derived macrophages forming crown-like aggregates along with a lowering in the hepatic expression of pro-inflammatory/pro-fibrogenic markers such as CCL2, IL-12, CD11b, α1-procollagen, TGF-β1, osteopontin, and galectin-3. Consistently, in vitro experiments showed that β-hydroxybutyrate supplementation reduced TREM2 and galectin-3 expression by cultured Raw 264.7 macrophages.
Conclusions: Altogether, these results indicate that ketogenic diet based on vegetal fat effectively improves MASH metabolic derangements and steatohepatitis, and it might represent a potential therapeutic strategy in this disease.
Keywords: gut dysbiosis; ketone bodies; liver fibrosis; liver inflammation; metabolic dysfunction-associated steatotic liver disease; non-alcoholic fatty liver disease; non-alcoholic steatohepatitis.
Copyright © 2025 Provera, Ramavath, Gadipudi, Vecchio, Caputo, Antonioli, Tini, Sheferaw, Reano, Filigheddu, Manfredi, Barberis, Cocolin, Ferrocino, Locatelli, Caprio, Tacke, Albano, Prodam and Sutti.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures




References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

