High Agreement Across Laboratories Between Different Alpha-Synuclein Seed Amplification Protocols
- PMID: 40237217
- PMCID: PMC12000918
- DOI: 10.1111/ene.70165
High Agreement Across Laboratories Between Different Alpha-Synuclein Seed Amplification Protocols
Abstract
Background: Seed amplification assays (SAA) detect alpha-synuclein (aSYN) pathology in patient biomatrices such as cerebrospinal fluid (CSF)-potentially even before clinical manifestations. As CSF-based SAA are approaching broader use in clinical trials and research, ensuring that different laboratories obtain the same results becomes increasingly important.
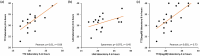
Methods: In this cross-laboratory, cross-aSYN-recombinant substrate and cross-protocol round-robin test, we compared SAA results from a common set of 38 CSF samples measured independently in four research laboratories of the German Center for Neurodegenerative diseases. Three laboratories (A-C) used an assay protocol adapted from Parchi's group at ISNB (Bologna, Italy); laboratory D used an assay protocol adapted from Amprion Inc. Two different manufacturers of aSYN protein were used as substrates for the SAA reaction.
Results: Qualitative results were identical in at least three of the four laboratories for 37 out of 38 samples (20 positive, 17 negative). Fleiss Kappa for all four laboratories was 0.751 (z = 12, p < 0.001). For each laboratory, agreement with laboratory A was > 92%. For the number of positive replicates, Fleiss Kappa was 0.45 for a score of zero positive replicates and 0.42 for a score of four positive replicates.
Conclusions: The qualitative SAA results showed a high level of agreement across research laboratories, aSYN monomers, and assay protocols. Small differences between laboratories were systematic, consistent with the notion that SAA reports biologically relevant properties. These results also underline that round-robin tests can be helpful in assessing and ensuring SAA quality across laboratories.
Keywords: RT‐QuIC; alpha‐synuclein; method validation; proficiency testing; ring trial.
© 2025 The Author(s). European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
Matthis Synofzik has received consultancy honoraria from Ionis, UCB, Prevail, Orphazyme, Biogen, Servier, Reata, GenOrph, AviadoBio, Biohaven, Zevra, Lilly, and Solaxa, all unrelated to the present manuscript. Luis Concha‐Marambio is an employee of Amprion, a biotechnology company with intellectual property to commercialize SAA technology for diagnostic purposes. He is an inventor of several SAA‐related patents and received grants from the Michael J. Fox Foundation for Parkinson's Research (MJFF‐021233, MJFF‐025017, MJFF‐024735). Kathrin Brockmann received Research Grants from the Michael J Fox Foundation for Parkinson's Research (“LRRK2 Kinase Activity”, “Influence of Inflammatory Profiles on PD Phenotype and Progression”, “Prevent Dementia in GBA‐associated PD”, MJFF PRKN/PINK1 Consortium, MJFF GBA1 Consortium on Joint Analysis, MJFF Grant Biology is the Disease), from the University of Tuebingen (“Endophenotyping of GBA‐PD”), from the German Society for Parkinson DPG, from the Health Forum Baden Wuerttemberg (“Predictive Diagnostic of immune‐associated diseases for personalized medicine”), from the Else Kröner Fresenius Stiftung (“ClinBrain”), and from the German Research Foundation DFG (“CORO‐TREND”). She serves on advisory boards for F. Hoffmann‐La Roche Ltd. and VanqaBio. She received speaker honoraria from Abbvie, Lundbeck, UCB and Zambon. Björn Falkenburger reports speaker honoraria from AbbVie, Stadapharm, Desitin, Zambon, and Bial, all unrelated to the present manuscript. The other authors declare no conflicts of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

