Small Extracellular Vesicle-Derived Nicotinamide Phosphoribosyltransferase (NAMPT) Induces Acyl-Coenzyme A Synthetase SLC27A4-Mediated Glycolysis to Promote Hepatocellular Carcinoma
- PMID: 40237223
- PMCID: PMC12000932
- DOI: 10.1002/jev2.70071
Small Extracellular Vesicle-Derived Nicotinamide Phosphoribosyltransferase (NAMPT) Induces Acyl-Coenzyme A Synthetase SLC27A4-Mediated Glycolysis to Promote Hepatocellular Carcinoma
Abstract
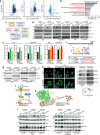
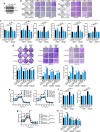
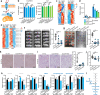
Tumour-derived small extracellular vesicles (sEV) are critical mediators within the tumour microenvironment (TME) and are known to regulate various metabolic pathways. In metastatic hepatocellular carcinoma (HCC), mass spectrometry protein analysis of HCC-derived sEV (HCC-sEV) identified an upregulation of nicotinamide phosphoribosyltransferase (NAMPT), a key enzyme in maintaining cellular nicotinamide adenine dinucleotide (NAD+) levels. Our study demonstrates that sEV-NAMPT enhances glycolysis, tumorigenesis, and metastasis in HCC. Specifically, sEV-NAMPT activates the NF-κB transcription factor through toll-like receptor 4 (TLR4), leading to elevated SLC27A4 expression. SLC27A4 functions primarily as a long-chain fatty acid transporter and acyl-CoA synthetase. Lipidomic and metabolomic analyses revealed a positive correlation between SLC27A4 and intracellular levels of triacylglycerol (TG) and dihydroxyacetone phosphate (DHAP). Increased TG levels enhance lipolysis via hepatic lipase and facilitate the conversion of glycerol-3-P to DHAP, an intermediate that bridges lipid metabolism and glycolysis. This study uncovers a novel regulatory axis involving sEV-NAMPT and SLC27A4 in glycolysis, independent of traditional fatty acid metabolism pathways. Clinically, targeting sEV-NAMPT with the inhibitor FK866 significantly inhibited tumour growth in various HCC in vivo models, highlighting the potential of sEV-NAMPT as both a biomarker and therapeutic target in HCC.
© 2025 The Author(s). Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Inhibition of Nicotinamide Phosphoribosyltransferase (NAMPT), an Enzyme Essential for NAD+ Biosynthesis, Leads to Altered Carbohydrate Metabolism in Cancer Cells.J Biol Chem. 2015 Jun 19;290(25):15812-15824. doi: 10.1074/jbc.M114.632141. Epub 2015 May 5. J Biol Chem. 2015. PMID: 25944913 Free PMC article.
-
FK866-induced NAMPT inhibition activates AMPK and downregulates mTOR signaling in hepatocarcinoma cells.Biochem Biophys Res Commun. 2015 Mar 6;458(2):334-40. doi: 10.1016/j.bbrc.2015.01.111. Epub 2015 Feb 3. Biochem Biophys Res Commun. 2015. PMID: 25656579
-
Extracellular Vesicle-Packaged ACSL4 Induces Hepatocyte Senescence to Promote Hepatocellular Carcinoma Progression.Cancer Res. 2024 Dec 2;84(23):3953-3966. doi: 10.1158/0008-5472.CAN-24-0832. Cancer Res. 2024. PMID: 39226516
-
Nicotinamide adenine dinucleotide (NAD+): essential redox metabolite, co-substrate and an anti-cancer and anti-ageing therapeutic target.Biochem Soc Trans. 2020 Jun 30;48(3):733-744. doi: 10.1042/BST20190033. Biochem Soc Trans. 2020. PMID: 32573651 Review.
-
Nicotinamide phosphoribosyltransferase (Nampt) in carcinogenesis: new clinical opportunities.Expert Rev Anticancer Ther. 2016 Aug;16(8):827-38. doi: 10.1080/14737140.2016.1190649. Epub 2016 Jul 5. Expert Rev Anticancer Ther. 2016. PMID: 27186719 Review.
References
-
- Arima, Y. , Liu W., Takahashi Y., Nishikawa M., and Takakura Y.. 2019. “Effects of Localization of Antigen Proteins in Antigen‐Loaded Exosomes on Efficiency of Antigen Presentation.” Molecular Pharmaceutics 16: 2309–2314. - PubMed
-
- Basak, M. , Chaudhary D. K., Takahashi R.‐U., et al. 2022. “Immunocyte Derived Exosomes: Insight Into the Potential Chemo‐Immunotherapeutic Nanocarrier Targeting the Tumor Microenvironment.” ACS Biomaterials Science & Engineering 9: 20–39. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

