Alleviation of Microglia Mediating Hippocampal Neuron Impairments and Depression-Related Behaviors by Urolithin B via the SIRT1-FOXO1 Pathway
- PMID: 40237232
- PMCID: PMC12000931
- DOI: 10.1111/cns.70379
Alleviation of Microglia Mediating Hippocampal Neuron Impairments and Depression-Related Behaviors by Urolithin B via the SIRT1-FOXO1 Pathway
Abstract
Aims: Conventional antidepressants exhibit limited efficacy and delayed onset. This study aimed to elucidate the antidepressant effects of urolithin B (UB) and its regulatory role in microglia-mediated hippocampal neuronal dysfunction.
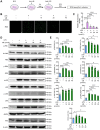
Methods: The mouse model of depression was established using both chronic unpredicted stress (CUS) and lipopolysaccharide (LPS) injection. The therapeutic efficacy of UB was assessed through behavioral paradigms. The microglia activation, cellular cytotoxicity and apoptosis levels, and underlying molecular mechanisms were delineated utilizing proteomics analysis, immunofluorescence staining, real-time PCR and Western blotting.
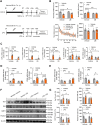
Results: UB efficiently alleviated depression-related behaviors, accompanied by suppressed microglia activation, neuroinflammation, changes of classic activation (M1)/alternative activation (M2) polarization and recovered sirtuin-1 (SIRT1) and forkhead box protein O1 (FOXO1) expression in the hippocampus. Additionally, UB reduced the cytotoxicity and apoptosis of HT22 cells and depression-related phenotypes treated by the cellular supernatant from LPS-incubated BV2 cells, which was mediated by the SIRT1-FOXO1 pathway. The proteomics analysis of the cellular supernatant content revealed abundant secreting proteins among the LPS/UB application.
Conclusion: This study confirmed that microglial SIRT1 mediates UB's antidepressant effects, positioning UB as a promising therapeutic candidate for depression by targeting neuroinflammatory pathways.
Keywords: FOXO1; SIRT1; cytotoxicity; depression; neuroinflammation; urolithin B.
© 2025 The Author(s). CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








References
-
- Marx W., Penninx B., Solmi M., et al., “Major Depressive Disorder,” Nature Reviews. Disease Primers 9, no. 1 (2023): 44. - PubMed
-
- Monroe S. M. and Harkness K. L., “Major Depression and Its Recurrences: Life Course Matters,” Annual Review of Clinical Psychology 18, no. 1 (2022): 329–357. - PubMed
-
- Chen M. H., Tu P. C., and Su T. P., Next Generation Antidepressants With Novel Mechanisms for Treatment Resistant Depression, 149–168. (Progress in Brain Research, 2023). - PubMed
MeSH terms
Substances
Grants and funding
- Shandong Provincial Key Medical and Health Laboratory of Research in neuropsychiatric disorders (Binzhou Medical University Hospital)
- ZR2021MH073/Shandong Provincial Natural Science Foundation
- ZR2022YQ65/Shandong Provincial Natural Science Foundation
- M-2023120/Shandong Traditional Chinese Medicine Scientific and Technological Project
- Q-2022121/Shandong Traditional Chinese Medicine Scientific and Technological Project
- Q-2023005/Shandong Traditional Chinese Medicine Scientific and Technological Project
- tsqn202211368/the Special Funds of the Taishan Scholars Project of Shandong Province
- 82171521/National Natural Science Foundation of China
- 82371539/National Natural Science Foundation of China
- Shandong Key Laboratory of Mental Disorders and Intelligent Control (Binzhou Medical University Hospital)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

