Distribution of Intranasally Administered rIL-10 Along the Olfactory Nerve and Perivascular Space After Intracerebral Hemorrhage
- PMID: 40237247
- PMCID: PMC12000929
- DOI: 10.1111/cns.70372
Distribution of Intranasally Administered rIL-10 Along the Olfactory Nerve and Perivascular Space After Intracerebral Hemorrhage
Abstract
Rationale: The utilization of anti-inflammatory therapy for treating brain diseases holds promise; however, research on intranasal administration of drug compounds remains limited. Quantitative data, particularly pharmacokinetics, are scant, and direct evidence of the distribution of intranasally administered recombinant interleukin 10 (rIL-10) within the brain is lacking.
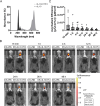
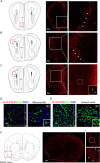
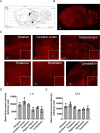
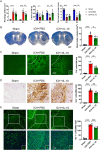
Methods: Employing fluorescent labeling, in vivo imaging, and confocal microscopy, we meticulously monitored the distribution and delivery pathways of intranasally administered rIL-10 in the brain.
Results and conclusions: Our findings demonstrate that rIL-10 can permeate the blood-brain barrier and reach the perihematomal area in the striatum of mice with intracerebral hemorrhage. Intranasally administered rIL-10 primarily targets the cerebral cortex, striatum, and thalamus, traversing the olfactory nerve pathway and perivascular space to access these brain regions. This mode of delivery effectively mitigated secondary brain injury after intracerebral hemorrhage. This study contributes to intranasal drug delivery research, offering compelling evidence to support the intranasal delivery of anti-inflammatory cytokines or alternative drug candidates for treating brain diseases.
Keywords: BBB; ICH; in vivo imaging; intranasal administration; rIL‐10.
© 2025 The Author(s). CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Long Y., Yang Q., Xiang Y., et al., “Nose to Brain Drug Delivery—A Promising Strategy for Active Components From Herbal Medicine for Treating Cerebral Ischemia Reperfusion,” Pharmacological Research 159 (2020): 104795. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

