Manganese-Catalyzed Enantioselective Dearomative Epoxidation of Naphthalenes with Aqueous Hydrogen Peroxide
- PMID: 40237300
- PMCID: PMC12184291
- DOI: 10.1002/anie.202504356
Manganese-Catalyzed Enantioselective Dearomative Epoxidation of Naphthalenes with Aqueous Hydrogen Peroxide
Abstract
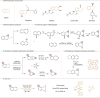
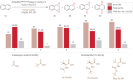
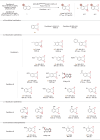
Arenes are abundantly occurring molecules of significant interest as versatile starting materials in organic reactions. Typically, oxidation of arenes yields planar molecules such as phenols and quinones. However, several iron dependent oxygenases can disrupt the aromaticity of arenes through oxidation and introduce C(sp3)─O stereogenic centers, resulting in precious enantioenriched epoxide or diol products. Emulating this enzymatic behavior with synthetic catalysts has met little success until now. Herein we describe a catalytic chemo- and enantioselective dearomative epoxidation of naphthalenes. The singular chemo- and enantioselectivity features of the reaction critically rely on a manganese catalyst that combines electron donating groups and steric demand on the ligand and activates hydrogen peroxide under mild conditions and short reaction times. Assisted with an N-protected amino acid, this catalyst epoxidizes a range of naphthalenes providing chemically versatile diepoxides in moderate to good yields and high levels of enantioselectivity. Straightforward elaboration gives diverse access to densely functionalized 3D structurally rich oxygenated molecules. The reaction constitutes a paradigmatical example of expedient access to stereochemically rich, valuable oxygenated molecules from readily available feedstocks, enabled by highly reactive yet selective biologically inspired oxidation catalysts.
Keywords: Arenes; Bioinspired catalysis; Enantioselectivity; Epoxidation; Hydrogen peroxide.
© 2025 The Author(s). Angewandte Chemie International Edition published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

