Disease Clearance in Ulcerative Colitis: A Narrative Review
- PMID: 40237360
- PMCID: PMC12269726
- DOI: 10.1002/ueg2.12714
Disease Clearance in Ulcerative Colitis: A Narrative Review
Abstract
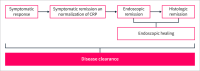
Ulcerative colitis (UC) is a chronic relapsing disease with significant associated risks such as colectomy, hospitalization, or colorectal cancer. A treat-to-target approach that mitigates disease activity and progression from an early stage is needed. The latest STRIDE II guidelines advocate for clinical and endoscopic remission as the main therapeutic targets in the management of UC; however, histological remission is increasingly being recognized as an important outcome. The concept of disease clearance, a composite outcome comprising clinical, endoscopic, and histological remission, has been proposed as a potential target for patients with UC and has been precisely defined by the International Organization for the Study of Inflammatory Bowel Disease, with the aim of standardizing its use in clinical practice and research. Despite challenges, including variable standardized definitions and uncertainties regarding the timing of reaching different definitions of remission, disease clearance corresponds to comprehensive disease control, and its use as an outcome could help clinicians to better evaluate the actual status of the disease. Furthermore, achieving disease clearance may be related to an improved disease course, positive long-term outcomes, and an improvement in health-related quality of life. Real-world evidence supports the feasibility of achieving disease clearance with various treatment modalities, including vedolizumab, the only gut-selective antilymphocyte trafficking drug. The aim of this narrative review is to explore the concept of disease clearance in patients with disease clearance, mainly focusing on trials evaluating vedolizumab but also other biologics.
Keywords: STRIDE; course; disease modification; histological healing; mucosal healing; progression; remission; target; therapy; vedolizumab.
© 2025 The Author(s). United European Gastroenterology Journal published by Wiley Periodicals LLC on behalf of United European Gastroenterology.
Conflict of interest statement
Silvio Danese reports receiving lecture fees from and serving as a consultant for AbbVie, Allergan, Biogen, Boehringer Ingelheim, Celgene, Celltrion, Ferring, Hospira, Johnson & Johnson, Merck, Merck Sharp & Dohme, Mundipharma, Pfizer, Sandoz, Takeda, TiGenix, UCB, and Vifor. Laurent Peyrin‐Biroulet reports receiving consulting fees from AbbVie, Abivax, Adacyte, Alimentiv, Amgen, Applied Molecular Transport, Arena, Banook, Biogen, Bristol Myers Squibb, Celltrion, Connect Biopharm, Cytoki Pharma, Enthera, Ferring, Fresenius Kabi, Galapagos, Genentech, Gilead, GlaxoSmithKline, Gossamer Bio, IAC Image Analysis, Index Pharmaceuticals, Inotrem, Janssen, Medac, Mopac, Morphic, Merck Sharp & Dohme, Nordic Pharma, Novartis, Oncodesign Precision Medicine, ONO Pharma, OSE Immunotherapeuthics, Pandion Therapeutics, Par' Immune, Pfizer, Prometheus, Protagonist, Roche, Samsung, Sandoz, Sanofi, Satisfay, Takeda, Telavant, Theravance, Thermo Fischer, TiGenix, Tillots, Vectivbio, Ventyx, Viatris, and Ysopia; grants from Celltrion, Fresenius Kabi, Medac, Merck Sharp & Dohme, and Takeda; and lecture fees from AbbVie, Amgen, Arena, Biogen, Celltrion, Eli Lilly and Company, Ferring, Galapagos, Genentech, Gilead, Janssen, Medac, Merck Sharp & Dohme, Nordic Pharma, Pfizer, Sandoz, Takeda, Tillots, and Viatris. Vipul Jairath reports receiving consulting/advisory board fees from AbbVie, Alimentiv, Arena, Asahi Kasei Pharma, Asieris, AstraZeneca, Bristol Myers Squibb, Celltrion, Eli Lilly and Company, Ferring, Flagship Pioneering, Fresenius Kabi, Galapagos, Genentech, Gilead, GlaxoSmithKline, Janssen, Merck, Mylan, Pandion, Pendopharm, Pfizer, Protagonist, Reistone Biopharma, Roche, Sandoz, Second Genome, Takeda, Teva, Topivert, Ventyx, and Vividion; and speaker fees from AbbVie, Ferring, Fresenius Kabi, Galapagos, Janssen, Pfizer, Shire, and Takeda. Ferdinando D'Amico serves as a speaker for Galapagos, Janssen, Omega Pharma, Sandoz, and Takeda and as an advisory board member for AbbVie, Ferring, Galapagos, Janssen, and Nestlé. Fernando Magro has served as a speaker for AbbVie, Arena, Biogen, Bristol Myers Squibb, Eli Lilly and Company, Falk, Ferring, Hospira, Janssen, Laboratórios Vitoria, Merck Sharp & Dohme, Pfizer, Sandoz, Takeda, UCB, and Vifor. Shashi Adsul and Christian Agboton are employees of and hold stock/stock options in Takeda.
Figures
Similar articles
-
The impact of biological interventions for ulcerative colitis on health-related quality of life.Cochrane Database Syst Rev. 2015 Sep 22;2015(9):CD008655. doi: 10.1002/14651858.CD008655.pub3. Cochrane Database Syst Rev. 2015. PMID: 26393522 Free PMC article.
-
Cyclosporine A for induction of remission in severe ulcerative colitis.Cochrane Database Syst Rev. 2005 Jan 25;(1):CD004277. doi: 10.1002/14651858.CD004277.pub2. Cochrane Database Syst Rev. 2005. PMID: 15674937
-
Unfractionated or low-molecular weight heparin for induction of remission in ulcerative colitis.Cochrane Database Syst Rev. 2010 Oct 6;(10):CD006774. doi: 10.1002/14651858.CD006774.pub3. Cochrane Database Syst Rev. 2010. PMID: 20927749
-
Vedolizumab for induction and maintenance of remission in ulcerative colitis.Cochrane Database Syst Rev. 2014 Aug 8;2014(8):CD007571. doi: 10.1002/14651858.CD007571.pub2. Cochrane Database Syst Rev. 2014. PMID: 25105240 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
Cited by
-
The Herbal Pair Polygonum hydropiper L-Coptis chinensis Franch Attenuate DSS-Induced Ulcerative Colitis by Modulating Metabolism and Intestinal Flora.Food Sci Nutr. 2025 Jul 8;13(7):e70584. doi: 10.1002/fsn3.70584. eCollection 2025 Jul. Food Sci Nutr. 2025. PMID: 40630428 Free PMC article.
References
-
- Turner D., Ricciuto A., Lewis A., et al., “STRIDE‐II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat‐to‐Target Strategies in IBD,” Gastroenterology 160, no. 5 (2021): 1570–1583, 10.1053/j.gastro.2020.12.031. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical