Covalently Interlocked Electrode-Electrolyte Interface for High-Energy-Density Quasi-Solid-State Lithium-Ion Batteries
- PMID: 40237362
- PMCID: PMC12165079
- DOI: 10.1002/advs.202417143
Covalently Interlocked Electrode-Electrolyte Interface for High-Energy-Density Quasi-Solid-State Lithium-Ion Batteries
Abstract
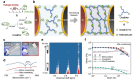
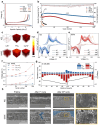
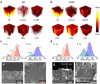
Quasi-solid-state batteries (QSSBs) are attracting considerable interest as a promising approach to enhance battery safety and electrochemical performance. However, QSSBs utilizing high-capacity active materials with substantial volume fluctuations, such as Si microparticle (SiMP) anodes and Ni-rich cathodes (NCM811), suffer from unstable interfaces due to contact loss during cycling. Herein, an in situ interlocking electrode-electrolyte (IEE) system is introduced, leveraging covalent crosslinking between acrylate-functionalized interlocking binders on active materials and crosslinkers within the quasi-solid-state electrolyte (QSSE) to establish a robust, interconnected network that maintains stable electrode-electrolyte contact. This IEE system addresses the limitations of liquid electrolyte and QSSE configurations, evidenced by low voltage hysteresis in (de)lithiation peaks over 200 cycles, stable interfacial resistance throughout cycling, and the absence of void formation. A pressure-detecting cell kit further confirms that the IEE system exhibits lower pressure changes during cycling without any voltage fluctuations from contact loss. Moreover, the SiMP||NCM811 full cell with the IEE system demonstrates superior electrochemical performance, and a bi-layer pouch cell configuration achieves an impressive energy density of 403.7 Wh kg-1/1300 Wh L-1, withstanding mechanical abuse tests such as folding and cutting, providing new insights into high-energy-density QSSBs.
Keywords: electrode–electrolyte interface; high‐energy‐density lithium‐ion batteries; interlocking system; quasi‐solid‐state batteries; silicon microparticle anodes.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- a) Ryu J., Song W. J., Lee S., Choi S., Park S., Adv. Funct. Mater. 2020, 30, 1902499;
- b) Cano Z. P., Banham D., Ye S. Y., Hintennach A., Lu J., Fowler M., Chen Z. W., Nat. Energy 2018, 3, 279.
-
- a) Wang Y., Cao G. Z., Adv. Mater. 2008, 20, 2251;
- b) Park C. M., Kim J. H., Kim H., Sohn H. J., Chem. Soc. Rev. 2010, 39, 3115. - PubMed
-
- a) Janek J., Zeier W. G., Nat. Energy 2023, 8, 230;
- b) Janek J., Zeier W. G., Nat. Energy 2016, 1, 16141;
- c) Zhang L., Wang S., Wang Q., Shao H. Y., Jin Z., Adv. Mater. 2023, 35, 202303355; - PubMed
- d) Wang S., Zhang L., Zeng Q. H., Guan J. Z., Gao H. Q., Zhang L. Y., Zhong J., Lai W. Y., Wang Q., Adv. Energy Mater. 2024, 14, 202302876.
-
- a) Huo H. Y., Jiang M., Bai Y., Ahmed S., Volz K., Hartmann H., Henss A., Singh C. V., Raabe D., Janek J., Nat. Mater. 2024, 23, 543; - PMC - PubMed
- b) Cangaz S., Hippauf F., Reuter F. S., Doerfler S., Abendroth T., Althues H., Kaskel S., Adv. Energy Mater. 2020, 10, 2001320;
- c) Wang S., Xiao S. J., Li S. H., Liu C., Cai H. N., Sun W. Q., Huang Z. D., Lai W. Y., Angew. Chem., Int. Ed. 2024, 63, 202412434. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
