Increased Atherosclerosis in HIV-Infected Humanized Mice Is Caused by a Single Viral Protein, Nef
- PMID: 40237461
- PMCID: PMC12308672
- DOI: 10.1093/infdis/jiaf192
Increased Atherosclerosis in HIV-Infected Humanized Mice Is Caused by a Single Viral Protein, Nef
Abstract
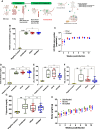
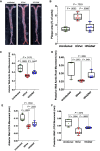
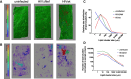
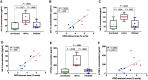
Antiretroviral therapy suppresses human immunodeficiency virus (HIV) replication, reverses immunodeficiency, and reduces AIDS-related symptoms, but non-AIDS comorbidities like cardiovascular diseases remain a major challenge for people with HIV (PWH). The pathogenic mechanisms driving these comorbidities are poorly understood. We previously showed that the HIV protein Nef contributes to chronic inflammation in PWH. Here, we explored Nef's role in HIV-associated atherosclerosis using a novel model: HIV-infected humanized mice expressing a gain-of-function mutant of proprotein convertase subtilisin/kexin type 9 (PCSK9) and fed a high-fat diet. Comparing atherosclerosis in uninfected mice to those infected with Nef-positive or Nef-deficient HIV-1, we found that Nef exacerbates atherosclerotic changes by increasing inflammation. These results identify Nef as a key driver of HIV-related atherosclerosis and provide a platform for testing therapeutic interventions targeting Nef to mitigate cardiovascular risks in PWH.
Keywords: HIV; Nef; atherosclerosis; humanized mice; inflammation.
© The Author(s) 2025. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

