Stabilized dengue virus 2 envelope subunit vaccine redirects the neutralizing antibody response to all E-domains
- PMID: 40237498
- PMCID: PMC12090738
- DOI: 10.1128/jvi.00229-25
Stabilized dengue virus 2 envelope subunit vaccine redirects the neutralizing antibody response to all E-domains
Abstract
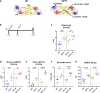
The four dengue virus (DENV) serotypes cause several hundred million infections annually. Several live-attenuated tetravalent dengue vaccines (LAVs) are at different stages of clinical testing and regulatory approval. A major hurdle faced by the two leading LAVs is uneven replication of vaccine serotypes stimulating a dominant response to one serotype at the expense of the other three, leading to the potential for vaccine antibody (Ab)-enhanced, more severe infections by wild-type (WT) DENV serotypes that fail to replicate in the vaccine. Protein subunit vaccines are a promising alternative since antigen dosing can be precisely controlled. However, DENV envelope (E) protein subunit vaccines have not performed well to date, possibly due to differences between the monomeric structure of soluble E and the E homodimer of the viral surface. Previously, we have combined structure-guided computational and experimental approaches to design and produce DENV2 E antigens that are stable homodimers at 37℃ and stimulate higher levels of neutralizing Abs (NAbs) than the WT E antigen in mice. The goal of this study was to evaluate if DENV2 E homodimers stimulate NAbs that target different epitopes on E protein compared to the WT E monomer. Using DENV4/2 chimeric viruses and Ab depletion methods, we mapped the WT E-elicited NAbs to simple epitopes on domain III of E. In contrast, the stable E homodimer stimulated a more complex response toward all three surface-exposed domains of the E protein. Our findings highlight the impact of DENV2 E oligomeric state on the quality and specificity of DENV NAbs and the promise of DENV E homodimers as subunit vaccines.IMPORTANCEThe ideal dengue virus (DENV) vaccine should elicit a balanced and highly protective immune response against all four DENV serotypes. Current tetravalent live-attenuated DENV vaccines have faced challenges due to uneven replication of vaccine virus strains stimulating a strong immune response to one serotype and weak responses to the other three. Protein subunit vaccines provide novel opportunities to stimulate a balanced response because dosing can be precisely controlled and independent of vaccine virus replication. Here, we compare immune responses elicited by a new DENV serotype 2 protein vaccine designed to match the structure of proteins on the viral surface. We find that proteins designed to match the viral surface stimulate better immune responses targeting multiple sites on the viral surface compared to previous protein vaccines. Our results justify further testing and development of these second-generation DENV protein subunit vaccines.
Keywords: dengue; envelope protein; epitope mapping; flavivirus; neutralizing antibodies; protein subunit vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Update of
-
Stabilized dengue virus 2 envelope subunit vaccine redirects the neutralizing antibody response to all E-domains.bioRxiv [Preprint]. 2025 Feb 13:2024.07.18.604114. doi: 10.1101/2024.07.18.604114. bioRxiv. 2025. Update in: J Virol. 2025 May 20;99(5):e0022925. doi: 10.1128/jvi.00229-25. PMID: 39990303 Free PMC article. Updated. Preprint.
Similar articles
-
Stabilized dengue virus 2 envelope subunit vaccine redirects the neutralizing antibody response to all E-domains.bioRxiv [Preprint]. 2025 Feb 13:2024.07.18.604114. doi: 10.1101/2024.07.18.604114. bioRxiv. 2025. Update in: J Virol. 2025 May 20;99(5):e0022925. doi: 10.1128/jvi.00229-25. PMID: 39990303 Free PMC article. Updated. Preprint.
-
Dimerization of Dengue Virus E Subunits Impacts Antibody Function and Domain Focus.J Virol. 2020 Aug 31;94(18):e00745-20. doi: 10.1128/JVI.00745-20. Print 2020 Aug 31. J Virol. 2020. PMID: 32611757 Free PMC article.
-
Toward a deeper understanding of dengue: novel method for quantification and isolation of envelope protein epitope-specific antibodies.mSphere. 2025 May 27;10(5):e0096124. doi: 10.1128/msphere.00961-24. Epub 2025 Apr 11. mSphere. 2025. PMID: 40214258 Free PMC article.
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
-
Next-generation Dengue Vaccines: Leveraging Peptide-Based Immunogens and Advanced Nanoparticles as Delivery Platforms.J Pharm Sci. 2024 Aug;113(8):2044-2054. doi: 10.1016/j.xphs.2024.05.010. Epub 2024 May 16. J Pharm Sci. 2024. PMID: 38761864 Review.
References
-
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GRW, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. doi:10.1038/nature12060 - DOI - PMC - PubMed
-
- Situation report No 24 - dengue epidemiological situation in the region of the Americas - epidemiological week 24. 2024. PAHO/WHO | Pan American Health Organization. Available from: https://www.paho.org/en/documents/situation-report-no-24-dengue-epidemio.... Retrieved 9 Jul 2024.
-
- Halstead S. 2019. Recent advances in understanding dengue. F1000Res 8:F1000 Faculty Rev-1279. doi:10.12688/f1000research.19197.1 - DOI
-
- Manoff SB, George SL, Bett AJ, Yelmene ML, Dhanasekaran G, Eggemeyer L, Sausser ML, Dubey SA, Casimiro DR, Clements DE, Martyak T, Pai V, Parks DE, Coller BAG. 2015. Preclinical and clinical development of a dengue recombinant subunit vaccine. Vaccine 33:7126–7134. doi:10.1016/j.vaccine.2015.09.101 - DOI - PubMed
-
- Manoff SB, Sausser M, Falk Russell A, Martin J, Radley D, Hyatt D, Roberts CC, Lickliter J, Krishnarajah J, Bett A, Dubey S, Finn T, Coller BA. 2019. Immunogenicity and safety of an investigational tetravalent recombinant subunit vaccine for dengue: results of a Phase I randomized clinical trial in flavivirus-naïve adults. Hum Vaccin Immunother 15:2195–2204. doi:10.1080/21645515.2018.1546523 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

