The development and evolution of arthropod tagmata
- PMID: 40237508
- PMCID: PMC12001983
- DOI: 10.1098/rspb.2024.2950
The development and evolution of arthropod tagmata
Abstract
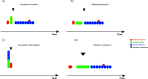
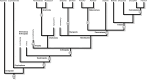
The segmented body is a hallmark of the arthropod body plan. Morphological segments are formed during embryogenesis, through a complex procedure involving the activation of a series of gene regulatory networks. The segments of the arthropod body are organized into functional units known as tagmata, and these tagmata are different among the arthropod classes (e.g. head, thorax and abdomen in insects). Based on embryological work on segment generation in a number of arthropod species, coupled with a survey of classical descriptions of arthropod development, I suggest a new framework for the evolution of arthropod tagmata. The ancestral condition involves three developmental tagmata: the pre-gnathal segments, a tagma that is formed within a pre-existing developmental field and a tagma that is formed through the activity of a segment-addition zone that may be embryonic or post-embryonic. These embryonic tagmata may fuse post-embryonically to generate more complex adult tagmata. This framework is consistent with the evolution of tagmosis seen in the early arthropod fossil record. It also calls for a re-thinking of the decades-old division of arthropod development into short-germ versus long-germ development, a re-thinking of questions of segment identity determination and the role of Hox genes in tagma differentiation.
Keywords: Hox genes; arthropods; body plan; segment identity; tagma.
Conflict of interest statement
I declare I have no competing interests.
Figures

References
-
- Fusco G, Minelli A. 2013. Arthropod segmentation and tagmosis. In Arthropod biology and evolution (eds Minelli A, Boxshall G, Fusco G), pp. 197–221. Berlin, Germany: Springer. (10.1007/978-3-642-36160-9_9) - DOI
-
- Minelli A. 2011. The Myriapoda. In Treatise on zoology: anatomy, taxonomy, biology. Leiden, The Netherlands: Brill.
-
- Schram FR, Koenemann S. 2022. Evolution and phylogeny of pancrustace - a story of scientific method. Oxford, UK: Oxford University Press.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
