Housing Temperature Impacts the Systemic and Tissue-Specific Molecular Responses to Cancer in Mice
- PMID: 40237521
- PMCID: PMC12001421
- DOI: 10.1002/jcsm.13781
Housing Temperature Impacts the Systemic and Tissue-Specific Molecular Responses to Cancer in Mice
Abstract
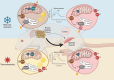
Background: Cancer cachexia, affecting up to 80% of patients with cancer, is characterized by muscle and fat loss with functional decline. Preclinical research seeks to uncover the molecular mechanisms underlying cachexia to identify potential targets. Housing laboratory mice at ambient temperature induces cold stress, triggering thermogenic activity and metabolic adaptations. Yet, the impact of housing temperature on preclinical cachexia remains unknown.
Methods: Colon 26 carcinoma (C26)-bearing and PBS-inoculated (Ctrl) mice were housed at standard (ST; 20°C-22°C) or thermoneutral temperature (TN; 28°C-32°C). They were monitored for body weight, composition, food intake and systemic factors. Upon necropsy, tissues were weighed and used for evaluation of ex vivo force and respiration, or snap frozen for biochemical assays.
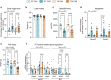
Results: C26 mice lost 7.5% body weight (p = 0.0001 vs. Ctrls), accounted by decreased fat mass (-35%, p < 0.0001 vs. Ctrls), showing mild cachexia irrespective of housing temperature. All C26 mice exhibited reduced force (-40%, p < 0.0001 vs. Ctrls) and increased atrogene expression (3-fold, p < 0.003 vs. Ctrls). Cancer altered white adipose tissue (WAT)'s functional gene signature (49%, p < 0.05 vs. Ctrls), whereas housing temperature reduced brown adipose tissue (BAT)'s (-78%, p < 0.05 vs. ST Ctrl). Thermogenic capacity measured by Ucp1 expression decreased upon cancer in both WAT and BAT (-93% and -63%, p < 0.0044 vs. Ctrls). Cancer-driven glucose intolerance was noted at ST (26%, p = 0.0192 vs. ST Ctrl), but restored at TN (-23%, p = 0.005 vs. ST C26). Circulating FGF21, GDF-15 and IL-6 increased in all C26 mice (4-fold, p < 0.009 vs. Ctrls), with a greater effect on IL-6 at TN (76%, p = 0.0018 vs. ST C26). Tumour and WAT Il6 mRNA levels remained unchanged, while cancer induced skeletal muscle (SkM) Il6 (2-fold, p = 0.0016 vs. Ctrls) at both temperatures. BAT Il6 was only induced in C26 mice at TN (116%, p = 0.0087 vs. ST C26). At the bioenergetics level, cancer increased SkM SERCA ATPase activity at ST (4-fold, p = 0.0108 vs. ST Ctrl) but not at TN. In BAT, O2 consumption enhanced in C26 mice at ST (119%, p < 0.03 vs. ST Ctrl) but was blunted at TN (-44%, p < 0.0001 vs. ST C26). Cancer increased BAT ATP levels regardless of temperature (2-fold, p = 0.0046 vs. Ctrls), while SERCA ATPase activity remained unchanged at ST and decreased at TN (-59%, p = 0.0213 vs. TN Ctrl).
Conclusions: In mild cachexia, BAT and SkM bioenergetics are susceptible to different housing temperatures, which influences cancer-induced alterations in glucose metabolism and systemic responses.
Keywords: bioenergetics; cancer cachexia; cold‐induced stress; thermogenic tissues; thermoneutrality.
© 2025 The Author(s). Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

