A Phase 1C, Open Label, Single Ascending Dose Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of DM199 Administered Intravenously with a Polyvinyl Chloride Bag in Adult Healthy Subjects and Adults Recently Taking Angiotensin-Converting Enzyme Inhibitors
- PMID: 40237565
- PMCID: PMC12130357
- DOI: 10.1002/cpdd.1534
A Phase 1C, Open Label, Single Ascending Dose Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of DM199 Administered Intravenously with a Polyvinyl Chloride Bag in Adult Healthy Subjects and Adults Recently Taking Angiotensin-Converting Enzyme Inhibitors
Abstract
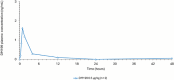
This small phase 1C, open-label, single ascending dose study evaluated the safety, tolerability, and pharmacokinetics (PKs) of DM199, a recombinant form of human tissue kallikrein-1 (KLK1). A small sample size of both healthy subjects and hypertensive adults recently taking angiotensin-converting enzyme inhibitors was studied. KLK1 has a known role in vasodilation and blood flow regulation, with potential implications for treatment of acute ischemic stroke (AIS) by focally enhancing cerebral perfusion. A total of 12 subjects were enrolled; 9 healthy subjects received escalating doses of DM199 (0.1-0.5 µg/kg), while 3 hypertensive subjects received the maximum tolerated dose of 0.5 µg/kg. Safety assessments indicated that DM199 was well tolerated, with mild adverse events reported, such as headache and flushing. No infusion-related hypotensive events occurred, and all subjects completed the study without significant clinical issues. The study was performed following prior PK analyses revealing that DM199 exposure was greater when administered with polyvinyl chloride infusion materials compared with polyolefin infusion materials. This study supports a revised dosing strategy for DM199 in the ongoing ReMEDy2 trial for AIS and highlights the need for careful consideration of the risk-benefit profile in the clinical context of AIS treatment.
Keywords: ACE inhibitors; DM199; pharmacokinetics; phase 1C clinical trial; safety; tolerability.
© 2025 The Author(s). Clinical Pharmacology in Drug Development published by Wiley Periodicals LLC on behalf of American College of Clinical Pharmacology.
Conflict of interest statement
M.G.: Board of Director and shareholder of DiaMedica Therapeutics, Inc. A.L.: Employee, DiaMedica Therapeutics, Inc. N.P.: Employee, DiaMedica Therapeutics, Inc. L.M.: Employee, DiaMedica Therapeutics, Inc.
Figures

References
-
- Regoli D, Gobeil F. Critical insights into the beneficial and protective actions of the kallikrein–kinin system. Vasc Pharmacol. 2015;64:1‐10. - PubMed
-
- Naicker S, Naidoo S, Ramsaroop R, Moodley D, Bhoola K. Tissue kallikrein and kinins in renal disease. Immunopharmacology. 1999;44(1‐2):183‐192. - PubMed
-
- Pérez‐Blanco FJ, Manzanares‐Olivares L, Domínguez‐Vicent JR, Moreno‐Terribas G, Rodríguez‐Cuartero A. Urinary kallikrein excretion in early diabetic nephropathy. Clin Nephrol. 1997;47(1):64‐65. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

