Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis
- PMID: 40237648
- PMCID: PMC12002006
- DOI: 10.1002/14651858.CD011689.pub4
Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis
Abstract
Rationale: Postpartum haemorrhage (PPH) is the leading cause of maternal mortality worldwide. Prophylactic uterotonic agents can prevent PPH. The current World Health Organization (WHO) recommendation for preventing PPH is 10 IU (international units) of intramuscular or intravenous oxytocin. Several uterotonics prevent PPH, but there remains uncertainty about the most effective agent with the fewest side effects. This is an update of a review first published in April 2018, and incorporates trustworthiness screening of eligible trials.
Objectives: To identify the most effective uterotonic agent(s) to prevent PPH with the fewest side effects, and generate a ranking according to their effectiveness and side effect profile.
Search methods: On 5 February 2024, we searched CENTRAL, MEDLINE, Embase and CINAHL in collaboration with the Cochrane Information Specialist.
Eligibility criteria: All randomised controlled trials (RCTs) or cluster-RCTs that compared the effectiveness and side effects of uterotonic agents with other uterotonic agents, placebo or no treatment for preventing PPH were eligible for inclusion. We screened eligible trials for trustworthiness. We included randomised trials published only as abstracts if we could retrieve sufficient information; we excluded quasi-randomised trials.
Outcomes: Primary outcomes were PPH ≥ 500 mL and PPH ≥ 1000 mL. Secondary outcomes included use of additional uterotonics, blood transfusion, vomiting, hypertension, and fever.
Risk of bias: We used RoB 1 to assess risk of bias.
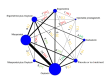
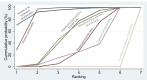
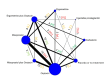
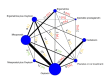
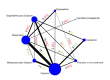
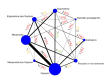
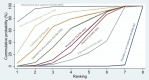
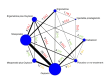
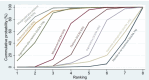
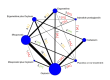
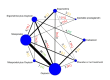
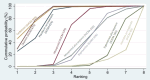
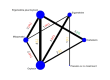
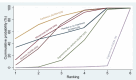
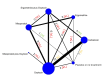
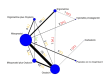
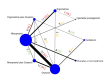
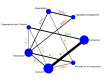
Synthesis methods: At least three review authors independently assessed trials for inclusion, trustworthiness, risk of bias, and certainty of evidence using GRADE. We estimated the relative effects and rankings for the primary and secondary outcomes. We reported primary outcomes for prespecified subgroups, stratified by mode of birth (caesarean versus vaginal), setting (hospital versus community), prior risk of PPH (high versus low), dose of misoprostol (≥ 600 μg versus < 600 μg), and regimen of oxytocin (bolus versus bolus plus infusion versus infusion only). We performed pairwise meta-analyses and network meta-analysis to determine the relative effects and rankings of all available agents.
Included studies: The network meta-analysis included 122 trials (121,931 women), involving seven uterotonic agents and placebo or no treatment, conducted across 48 high-, middle- and low-income countries. Most were in a hospital setting (115/122, 94%), with women having a vaginal birth (87/122, 71%).
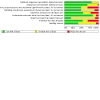
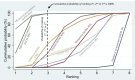
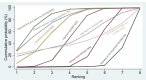
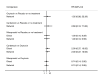
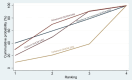
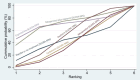
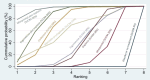
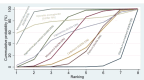
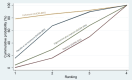
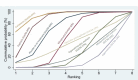
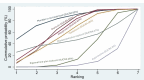
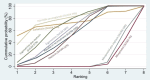
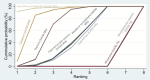
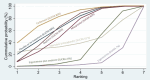
Synthesis of results: Relative effects from the network meta-analysis suggested that all agents, except injectable prostaglandins, for which data were limited, were effective for preventing PPH ≥ 500 mL compared with placebo or no treatment. The two highest-ranked agents were ergometrine plus oxytocin and misoprostol plus oxytocin. Compared with oxytocin, ergometrine plus oxytocin reduces PPH ≥ 500 mL (risk ratio (RR) 0.76, 95% confidence interval (CI) 0.64 to 0.90, high-certainty evidence), and misoprostol plus oxytocin probably reduces PPH ≥ 500 mL (RR 0.70, 95% CI 0.57 to 0.87; moderate-certainty evidence). Carbetocin (high-), injectable prostaglandins (moderate-) and ergometrine (low-certainty evidence) have similar effects compared with oxytocin. The evidence for misoprostol is very low certainty. All agents, except ergometrine and injectable prostaglandins, for which data were limited, were effective for preventing PPH ≥ 1000 mL compared with placebo or no treatment. Ergometrine plus oxytocin, and misoprostol plus oxytocin were the highest-ranked agents. Compared with oxytocin, carbetocin and injectable prostaglandins (both moderate-certainty evidence), and misoprostol plus oxytocin (low-certainty evidence) make little or no difference to PPH ≥ 1000 mL. Misoprostol may be less effective in preventing PPH ≥ 1000 mL compared with oxytocin (RR 1.24, 95% CI 1.06 to 1.46; low-certainty evidence). The certainty of evidence for ergometrine and ergometrine plus oxytocin was very low. Compared with oxytocin, misoprostol plus oxytocin probably reduces the use of additional uterotonics (RR 0.55, 95% CI 0.42 to 0.72, moderate-certainty evidence), and carbetocin (RR 0.74, 95% CI 0.59 to 0.94; low-certainty evidence), and ergometrine plus oxytocin may reduce the use of additional uterotonics (RR 0.68, 95% CI 0.56 to 0.83; low-certainty evidence). Misoprostol (low-certainty evidence) makes little or no difference to this outcome. Misoprostol plus oxytocin probably reduces the risk of needing a blood transfusion (RR 0.40, 95% CI 0.28 to 0.58; moderate-certainty-evidence), and ergometrine plus oxytocin may reduce the risk of blood transfusion compared with oxytocin (RR 0.73, 95% CI 0.56 to 0.96, low-certainty evidence). Carbetocin (moderate-certainty evidence) and misoprostol (low-certainty evidence) probably make little or no difference to this outcome compared with oxytocin. All uterotonic agents, except for carbetocin, were associated with increased risks of side effects compared with oxytocin. Misoprostol may increase the likelihood of nausea, vomiting and fever, and probably increases the risk of diarrhoea. Injectable prostaglandins may increase the likelihood of diarrhoea. Ergometrine probably increases the likelihood of nausea and vomiting, and may increase the likelihood of hypertension, headache, and diarrhoea. Ergometrine plus oxytocin may increase the likelihood of nausea, vomiting, and diarrhoea. Misoprostol plus oxytocin probably increases the likelihood of nausea, vomiting and diarrhoea, and may increase the likelihood of fever. Analyses of the prespecified subgroups did not reveal important subgroup differences. Evidence for outcomes not presented above but reported in the summary of findings tables was very low certainty.
Authors' conclusions: Most agents are effective for preventing PPH when compared with placebo or no treatment. Ergometrine plus oxytocin, and misoprostol plus oxytocin may be more effective than the current standard oxytocin. All agents, except for carbetocin, are associated with an increased risk of some side effects compared with oxytocin.
Funding: Supported by UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), a cosponsored programme executed by the WHO (Award No. HQHRP2220228-22.1-74309).
Registration: Cochrane Library; Registration number: CD011689 and protocol [and previous versions] available via DOI: 10.1002/14651858.CD011689 [DOI: 10.1002/14651858.CD011689.pub3 and DOI: 10.1002/14651858.CD011689.pub2].
Copyright © 2025 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
Ioannis D Gallos (IDG): was a co‐applicant to the UK National Institute for Health Research HTA Project Award 14/139/17 entitled
Idnan Yunas (IY): none known
Adam Devall (AD): none known
Marecelina Podesek (MP): none known
Malcolm J Price (MJP): was a co‐applicant to the UK National Institute for Health Research HTA Project Award 14/139/17 entitled
Aurelio Tobias: none known
Olufemi T Oladapo (OTO): led the updating of
Arri Coomarasamy (AC): was the Chief Investigator of UK National Institute for Health Research HTA Project Award 14/139/17 entitled
Figures

















Update of
-
Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis.Cochrane Database Syst Rev. 2018 Dec 19;12(12):CD011689. doi: 10.1002/14651858.CD011689.pub3. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2025 Apr 16;4:CD011689. doi: 10.1002/14651858.CD011689.pub4. PMID: 30569545 Free PMC article. Updated.
References
-
- Say L, Chou D, Gemmill A, Tunçalp O, Moller A, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Global Health 2014;2(6):e323-e333. - PubMed
-
- Cresswell J. Trends in Maternal Mortality 2000 to 2020 : Estimates by WHO, UNICEF, UNFPA, World Bank Group and UNDESA/Population Division. WHO, 2023.
-
- Penney G, Brace V. Near miss audit in obstetrics. Current Opinion in Obstetrics & Gynecology 2007;19:145-50. - PubMed
-
- Souza JP, Gülmezoglu AM, Vogel J, Carroli G, Lumbiganon P, Qureshi Z, et al. Moving beyond essential interventions for reduction of maternal mortality (the WHO Multicountry Survey on Maternal and Newborn Health): a cross-sectional study. Lancet 2013;381:1747-55. - PubMed
-
- WHO. WHO Recommendations: Uterotonics for the Prevention of Postpartum Haemorrhage. Geneva: Department of Reproductive Health, World Health Organization, 2018. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

