Interplay of Mycobacterium abscessus and Pseudomonas aeruginosa in experimental models of coinfection: Biofilm dynamics and host immune response
- PMID: 40237819
- PMCID: PMC12064063
- DOI: 10.1080/21505594.2025.2493221
Interplay of Mycobacterium abscessus and Pseudomonas aeruginosa in experimental models of coinfection: Biofilm dynamics and host immune response
Abstract
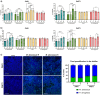
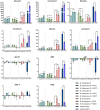
The incidence of infection by nontuberculous mycobacteria, mainly Mycobacterium abscessus, is increasing in patients with cystic fibrosis and other chronic pulmonary diseases, leading to an accelerated lung function decline. In most cases, M. abscessus coinfects Pseudomonas aeruginosa, the most common pathogen in these conditions. However, how these two bacterial species interact during infection remains poorly understood. This study explored their behaviour in three relevant pathogenic settings: dual-species biofilm development using a recently developed method to monitor individual species in dual-species biofilms, coinfection in bronchial epithelial cells, and in vivo coinfection in the Galleria mellonella model. The results demonstrated that both species form stable mixed biofilms and reciprocally inhibit single-biofilm progression. Coinfections in bronchial epithelial cells significantly decreased cell viability, whereas in G. mellonella, coinfections induced lower survival rates than individual infections. Analysis of the immune response triggered by each bacterium in bronchial epithelial cell assays and G. mellonella larvae revealed that P. aeruginosa induces the overexpression of proinflammatory and melanization cascade responses, respectively. In contrast, M. abscessus and P. aeruginosa coinfection significantly inhibited the immune response in both models, resulting in worse consequences for the host than those generated by a single P. aeruginosa infection. Overall, this study highlights the novel role of M. abscessus in suppressing immune responses during coinfection with P. aeruginosa, emphasizing the clinical implications for the management of cystic fibrosis and other pulmonary diseases. Understanding these interactions could inform the development of new therapeutic strategies to mitigate the severity of coinfections in vulnerable patients.
Keywords: Dual-species biofilm; Galleria mellonella; immunosuppression; nontuberculous mycobacteria; pulmonary epithelia.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
