Predictive Factors for Super Responder Status and Long-Term Effectiveness of Guselkumab in Psoriasis: A Multicenter Retrospective Study
- PMID: 40238056
- PMCID: PMC12033149
- DOI: 10.1007/s13555-025-01394-2
Predictive Factors for Super Responder Status and Long-Term Effectiveness of Guselkumab in Psoriasis: A Multicenter Retrospective Study
Abstract
Introduction: Psoriasis is a chronic inflammatory skin disorder, affecting around 2-3% of the global population. The IL-23/Th17 signaling pathway plays a critical role in disease progression. Guselkumab, an IL-23p19 monoclonal antibody, has shown substantial efficacy in clinical trials for treating moderate-to-severe plaque psoriasis. However, preliminary identification of super responders (SRe: patients achieving PASI 100 at week 20) can help optimize treatment strategies. This study aims to identify predictive factors for SRe status in patients receiving guselkumab therapy for psoriasis and to evaluate long-term effectiveness in the entire cohort and both SRe and non-super-responder (nSRe) groups to understand whether SRe status is also a predictor of long-term response to guselkumab in a real-world setting.
Methods: A retrospective longitudinal study was conducted at ten Italian centers between January and October 2024. Data from 1008 patients treated with guselkumab for at least 20 weeks were analyzed. Patients were classified as SRe (PASI 100 at week 20) and nSRe. Baseline clinical and anthropometric profiles, comorbidities, and treatment history were collected. Efficacy was evaluated using PASI scores. Logistic regression analysis was performed to identify predictive factors for achieving SRe status.
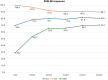
Results: Of 1008 patients, 581 (57.6%) were classified as SRe, while 427 (42.4%) were nSRe. SRe patients were more likely to be bio-naïve and had lower baseline PASI scores and comorbidities such as obesity, hypertension, and diabetes. Multivariate logistic regression identified obesity, prior biologic therapy, and a higher baseline PASI as negative predictors for SRe status. Guselkumab demonstrated significant long-term efficacy, with SRe patients achieving sustained PASI 100 in 85% at year 4 and 83.4% at year 5 compared to 54% and 59.2% in nSRe patients, respectively.
Conclusions: Our study highlights the importance of identifying patients most likely to achieve PASI 100 early in treatment. Factors such as obesity, prior biologic experience, and baseline PASI contribute to predicting complete skin clearance, which can guide clinical decision-making and enable personalized treatment strategies.
Keywords: Guselkumab; Long-term efficacy; Predictive factors; Psoriasis; Super responders.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Marco Galluzzo and Marina Talamonti declare to have acted as speakers and/or consultants for AbbVie, Almirall, Eli-Lilly, Johnson & Johnson, LeoPharma, Novartis and Sanofi, outside the submitted work. Luca Bianchi declares to have acted as a speaker and/or consultant for AbbVie, Almirall, Eli-Lilly, Johnson & Johnson, LeoPharma, Novartis, Pfizer, Sanofi and UCB outside the submitted work. Lorenzo Marcelli declares no conflict of interests. Anna Balato has served as consultant and/or has received fees from: AbbVie, Almirall, Amgen,Boehringer Ingelheim, BMS, Leo-Pharma, Eli-Lilly, Johnson & Johnson, Novartis, Sanofi and UCB, outside the submitted work. Dario Buononato declares no conflict of interests. Nicoletta Bernardini and Nevena Skroza declare to have acted as speakers and/or consultants for AbbVie, Almirall, Eli-Lilly, Johnson & Johnson, LeoPharma, Novartis and UCB, outside the submitted work. Giacomo Caldarola has received consulting fees, honoraria and support for attending meetings from Abbvie, Eli-Lilly, Johnson & Johnson, UCB, Novartis and Leopharma. Clara De Simone has received support for consulting fees, honoraria and support for attending meetings from Abbvie, Eli-Lilly, Johnson & Johnson, UCB, Leopharma, Sanofi, Almirall, Boehringer Ingelheim and Bristol Myer Squibb. Eleonora De Luca declares no conflict of interests. Francesco Loconsole declares no conflict of interests. Edoardo Mortato declares no conflict of interests. Felice Primavera declares no conflict of interests. Viviana Lora declares no conflict of interests. Claudio Bonifati declares no conflict of interests. Matteo Megna declares to have acted as speakers and/or consultants for AbbVie, Amgen, Almirall, Eli-Lilly, Janssen-Cilag, LeoPharma, Novartis, Eli Lilly, UCB, outside the submitted work. Luca Potestio e Nello Tommasino declares no conflict of interests. Serena Lembo declares to have acted as speakers and/or consultants for Abbvie, Eli Lilly, Sanofi, Novartis, Janssen, LeoPharma outside the submitted work. Annunziata Raimondo declares to have acted as a speaker and/or consultant for Almirall, Janssen, Pfizer, Abbvie, and Novartis outside the submitted work. Maria Esposito has served as speaker/consultant for Abbvie, Amgen, Almirall, Eli Lilly, Janssen, Leopharma, Novartis, Pfizer, Sanofi, UCB. Maria Concetta Fargnoli has served as consultant/advisor, received speaker honoraria and/or grants, and/or is investigator for AMGEN, Almirall, Abbvie, Boehringer-Ingelheim, BMS, Galderma, Kyowa Kyrin, Incyte, LEO Pharma, Pierre Fabre, UCB, Lilly, Pfizer, Janssen, MSD, Novartis, Sanofi, Regeneron, Sun Pharma, Takeda. Anna Campanati has served as speaker/consultant for Abbvie, Almirall, Amgen, BMS, Difa-Cooper, Eli Lilly, Janssen, Leopharma, Mertz, Novartis, Pfizer, Sanofi, UCB. Tommaso Bianchelli has served as speaker/consultant for Abbvie, Almirall, Amgen, Janssen, Novartis, Pfizer, Sanofi, UCB. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Anna Campanati is an Editorial Board member of Dermatology and Therapy. Anna Campanati was not involved in the selection of peer reviewers for the manuscript. Ethical Approval: The study was conducted in accordance with the 1975 Declaration of Helsinki ethical standards. According to Italian law, formal ethical committee approval is not required for this type of study.
Figures


References
-
- Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker JNWN. Psoriasis. Lancet. 2021;397:1301–15. - PubMed
-
- Oppmann B, Lesley R, Blom B, et al. Novel P19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–25. - PubMed
-
- Blauvelt A, Papp KA, Griffiths CEM, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405–17. - PubMed
LinkOut - more resources
Full Text Sources

