Proteomic Insights into Human Limbal Epithelial Progenitor-Derived Small Extracellular Vesicles
- PMID: 40238075
- PMCID: PMC12316787
- DOI: 10.1007/s12015-025-10877-w
Proteomic Insights into Human Limbal Epithelial Progenitor-Derived Small Extracellular Vesicles
Abstract
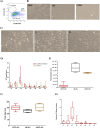
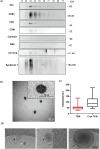
Limbal epithelial stem/progenitor cells (LEPC), supported by limbal mesenchymal stromal cells (LMSC) and limbal melanocytes (LM) within a specialized niche, are responsible for maintaining the corneal epithelium. Small extracellular vesicles (sEV) emerged as critical mediators of intercellular communication in various stem cell niches, yet their role in maintaining human limbal niche homeostasis remains poorly understood. In this study, tangential flow filtration and size exclusion chromatography were used to isolate sEV from LEPC-, LMSC- and LM-conditioned media. The isolated sEV from LEPC exhibited properties characteristic for sEV as confirmed by nanoparticle tracking analysis for size and concentration, by electron microscopy for morphology, and by western blot analysis of canonical EV markers including the cell-specific protein (cytokeratin 17/19). Quantitative and comparative proteomic profiling revealed distinct molecular signatures of LEPC-derived sEV, enriched in factors associated with keratinocyte development, extracellular matrix organization, and niche regulation. These findings suggest that LEPC-derived sEV may serve as important signaling mediators within the limbal niche microenvironment, though additional studies are needed to determine their specific functional roles in maintaining niche homeostasis.
Keywords: Exosomes; Limbal epithelial progenitor cells; Limbal melancoytes; Limbal mesenchymal stromal cells; Limbal stem cell niche; Proteomics; Small extracellular vesicles.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical Approval: The Institutional Review Board of the Medical Faculty of the University of Freiburg (25/20) approved experiments. Informed Consent: Informed consent to corneal donation had been obtained from the donors or their relatives. Consent to Participate: Not applicable. Consent to Publish: Not applicable. Competing interests: The authors declare no conflict of interest.
Figures


Similar articles
-
Enrichment, Characterization, and Proteomic Profiling of Small Extracellular Vesicles Derived from Human Limbal Mesenchymal Stromal Cells and Melanocytes.Cells. 2024 Apr 4;13(7):623. doi: 10.3390/cells13070623. Cells. 2024. PMID: 38607062 Free PMC article.
-
Efficient Isolation and Functional Characterization of Niche Cells from Human Corneal Limbus.Int J Mol Sci. 2022 Mar 2;23(5):2750. doi: 10.3390/ijms23052750. Int J Mol Sci. 2022. PMID: 35269891 Free PMC article.
-
P-Cadherin Is Expressed by Epithelial Progenitor Cells and Melanocytes in the Human Corneal Limbus.Cells. 2022 Jun 20;11(12):1975. doi: 10.3390/cells11121975. Cells. 2022. PMID: 35741104 Free PMC article.
-
A systematic review on the effects of ROCK inhibitors on proliferation and/or differentiation in human somatic stem cells: A hypothesis that ROCK inhibitors support corneal endothelial healing via acting on the limbal stem cell niche.Ocul Surf. 2023 Jan;27:16-29. doi: 10.1016/j.jtos.2022.12.008. Epub 2022 Dec 28. Ocul Surf. 2023. PMID: 36586668
-
Overcoming challenges in MSC-sEV therapeutics: insights and advances after a decade of research.Cytotherapy. 2025 Jul;27(7):843-848. doi: 10.1016/j.jcyt.2025.03.505. Epub 2025 Mar 20. Cytotherapy. 2025. PMID: 40243980 Review.
Cited by
-
Exosomes as Future Therapeutic Tools and Targets for Corneal Diseases.Cells. 2025 Jun 23;14(13):959. doi: 10.3390/cells14130959. Cells. 2025. PMID: 40643480 Free PMC article. Review.
References
-
- Cotsarelis, G., Cheng, S. Z., Dong, G., Sun, T. T., & Lavker, R. M. (1989). Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: Implications on epithelial stem cells. Cell,57, 201–209. 10.1016/0092-8674(89)90958-6 - PubMed
-
- Ordonez, P., & Di Girolamo, N. (2012). Limbal epithelial stem cells: Role of the niche microenvironment. Stem Cells,30, 100–107. 10.1002/stem.794 - PubMed
-
- Dziasko, M. A., Tuft, S. J., & Daniels, J. T. (2015). Limbal melanocytes support limbal epithelial stem cells in 2D and 3D microenvironments. Experimental Eye Research,138, 70–79. 10.1016/j.exer.2015.06.026 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

