Guideline-concordant treatment among adolescents and young adults with acute lymphoblastic leukemia
- PMID: 40238217
- PMCID: PMC12121638
- DOI: 10.1093/jncics/pkaf033
Guideline-concordant treatment among adolescents and young adults with acute lymphoblastic leukemia
Abstract
Background: Individuals diagnosed with acute lymphoblastic leukemia (ALL) between adolescents and young adults aged 15-39 years face poor survival and unique challenges. We evaluated facility-level factors and guideline-concordant care among adolescents and young adults with ALL at National Cancer Institute Community Oncology Research Program (NCORP) practices.
Methods: We assembled a retrospective cohort of adolescents and young adults aged 15-39 years with ALL treated at participating NCORPs between 2012 and 2016. NCORPs abstracted patient data and completed facility-level questionnaires for each clinical facility (study-defined criteria). The central review committee adjudicated whether treatment was concordant with adolescent and young adult-specific National Comprehensive Cancer Network ALL guidelines (ie, pediatric-inspired therapy or clinical trial). Guideline-concordant care was described by age, facility model (adult/internal medicine, pediatric, mixed [pediatric services within a general hospital]), and average annual adolescents and young adult ALL volume. Generalized linear mixed effects models estimated the odds of guideline-concordant care.
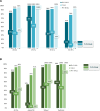
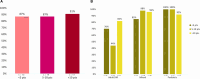
Results: Adolescents and young adults receiving guideline-concordant care were younger (n = 196; median = 19.5 years) than those who did not (n = 31; median = 32.1 years). Guideline-concordant care was observed in many adolescents and young adults aged 22-39 years (68.8%), and nearly universal in those aged 15-21 years. In multivariable analyses, adolescents and young adults at adult/internal medicine clinical facilities had lower odds of guideline-concordant care (odds ratio = 0.02, 95% confidence interval = 0.0 to 0.18); there was no statistically significant association between annual adolescent and young ALL volume and receiving guideline-concordant care. Guideline-concordant care was observed more often in adult/internal medicine and/or mixed clinical facilities with communication between adult or pediatric counterparts, adolescents and young adult ALL clinical pathways, and/or adolescent and young adult-specific meetings.
Conclusion: Guideline-concordant care among adolescents and young adults with ALL (specifically pediatric-inspired therapy) at NCORPs is associated with facility model (adult/internal medicine) but not adolescent and young adult ALL volume. Strategies to improve guideline-concordant care could include facilitating communication and clinical pathways at adult/internal medicine clinical facilities treating adolescent and young adult ALL.
© The Author(s) 2025. Published by Oxford University Press.
Conflict of interest statement
S.L. reports advisory board participation for Novartis and Amgen. E.C. reports sponsored research with Servier and advisory board for Jazz Pharmaceuticals. D.D. has been a consultant for Tempus, Amgen, Day One Bio, and Y-mAs Therapeutics. A.A. has received honoraria for consulting from Kite, Pfizer, Servier, and Takeda as well as research support from OBI, Amgen, Glycomimetics, Pfizer, Seattle Genetics/Pfizer, Abbvie, KURA, Servier, Immunogen/Abbvie, Incyte, BEAM, and Kite. All other authors report no conflicts of interest.
Figures

References
-
- Department of Health and Human Services, National Institutes of Health. Research to Reduce Morbidity and Improve Care for Pediatric, and Adolescent and Young Adult (AYA) Cancer Survivors. 2021. https://grants.nih.gov/grants/guide/rfa-files/rfa-ca-20-028.html
MeSH terms
Grants and funding
- U10CA180886/Children's Oncology Group
- NCI Community Oncology Research Program
- U10CA180899/Children's Oncology Group
- UG1 CA189974/CA/NCI NIH HHS/United States
- Children's Oncology Group
- UG1CA189974/SWOG
- UG1CA189828/ECOG-ACRIN
- U10 CA180899/CA/NCI NIH HHS/United States
- U10 CA180886/CA/NCI NIH HHS/United States
- UG1 CA189828/CA/NCI NIH HHS/United States
- UG1 CA189955/CA/NCI NIH HHS/United States
- UG1CA189955/Research Base
- UG1CA189823/Alliance for Clinical Trials in Oncology
- UG1 CA189823/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
