The role of epicardial adipose tissue remodelling in heart failure with preserved ejection fraction
- PMID: 40238568
- PMCID: PMC12160812
- DOI: 10.1093/cvr/cvaf056
The role of epicardial adipose tissue remodelling in heart failure with preserved ejection fraction
Abstract
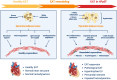
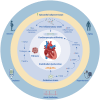
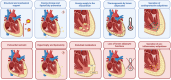
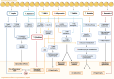
Heart failure with preserved ejection fraction (HFpEF) is a growing global health problem characterized by high morbidity and mortality, with limited effective therapies available. Obesity significantly influences haemodynamic and structural changes in the myocardium and vasculature, primarily through the accumulation and action of visceral adipose tissue. Particularly, epicardial adipose tissue (EAT) contributes to HFpEF through inflammation and lipotoxic infiltration of the myocardium. However, the precise signalling pathways leading to diastolic stiffness in HFpEF require further elucidation. This review explores the dynamic role of EAT in health and disease. Drawing upon insights from studies in other conditions, we discuss potential EAT-mediated inflammatory pathways in HFpEF and how they may contribute to functional and structural myocardial and endothelial derangements, including intramyocardial lipid infiltration, fibrosis, endothelial dysfunction, cardiomyocyte stiffening, and left ventricular hypertrophy. Lastly, we propose potential targets for novel therapeutic avenues.
Keywords: Epicardial adipose tissue; Heart failure with preserved ejection fraction; Inflammation; Inflammatory pathways.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: none declared.
Figures
References
-
- Shah SJ, Borlaug BA, Kitzman DW, McCulloch AD, Blaxall BC, Agarwal R, Chirinos JA, Collins S, Deo RC, Gladwin MT, Granzier H, Hummel SL, Kass DA, Redfield MM, Sam F, Wang TJ, Desvigne-Nickens P, Adhikari BB. Research priorities for heart failure with preserved ejection fraction: National Heart, Lung, and Blood Institute Working Group Summary. Circulation 2020;141:1001–1026. - PMC - PubMed
-
- Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2017;14:591–602. - PubMed
-
- McDonagh TA, Metra M, Adamo M, Baumbach A, Böhm M, Burri H, Čelutkiene J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gardner RS, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Piepoli MF, Price S, Rosano GMC, Ruschitzka F, Skibelund AK; ESC Scientific Document Group . 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:4901. - PubMed
-
- Redfield MM, Borlaug BA. Heart failure with preserved ejection fraction. JAMA 2023;329:827. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

