Validation of new equipment for SARS-CoV-2 diagnosis in Ecuador: Detection of the virus and antibodies generated by disease and vaccines with one POC device
- PMID: 40238804
- PMCID: PMC12002511
- DOI: 10.1371/journal.pone.0321794
Validation of new equipment for SARS-CoV-2 diagnosis in Ecuador: Detection of the virus and antibodies generated by disease and vaccines with one POC device
Abstract
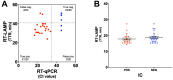
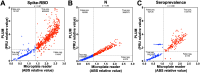
The COVID-19 pandemic underscored the critical need to enhance screening capabilities and streamline diagnosis. Point-of-care (POC) tests offer a promising solution by decentralizing testing. We aimed to validate the PLUM device (LSK Technologies Inc.), a portable optical reader, to detect SARS-CoV-2 RNA using direct RT-LAMP targeting the ORF1a and E1 genes and patient antibodies by ELISA. The direct RT-LAMP assays employ nasopharyngeal swabs and bypass RNA extraction protocols through a brief chemical and physical lysis step. Test sensitivity and specificity were assessed against gold-standard detection methods in laboratory and field conditions. For samples with Ct values below 25, direct RT-LAMP showed 83% sensitivity and 90% specificity under laboratory conditions and 91% sensitivity and 92% specificity under field conditions. The nucleocapsid antigen antibody assay had 99% positive percent agreement (PPA) and 97% negative percent agreement (NPA), outperforming spike-RBD antigen (98% PPA, 92% NPA) and seroprevalence (98% PPA, 88% NPA) under laboratory conditions. Under field conditions, similar results were found for antibody detection for the nucleocapsid antigen (93% PPA; 100% NPA), spike-RBD (100% PPA; 94% NPA), and seroprevalence (90% PPA; 94% NPA). This study validated the PLUM device as a dual POC tool for direct RT-LAMP-based SARS-CoV-2 and ELISA-based COVID-19 antibody detection.
Copyright: © 2025 Villota et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
Yuxiu Guo, Seray Cicek, and Keith Pardee are co-inventors of the PLUM device and co-founders of LSK Technologies, Inc. (acquired by Nicoya Life Science Inc.). Testing and data analysis was performed independently by the team in Ecuador without input from these authors. No other commercial declarations are relevant to this study. Anne-Claude Gingras receives funds from a research contract with Providence Therapeutics Holdings, Inc. for other projects. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures



References
-
- WHO. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on Nov 19, 2024).
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

