Earlier onset of chemotherapy-induced neuropathic pain in females by ICAM-1-mediated accumulation of perivascular macrophages
- PMID: 40238872
- PMCID: PMC12002127
- DOI: 10.1126/sciadv.adu2159
Earlier onset of chemotherapy-induced neuropathic pain in females by ICAM-1-mediated accumulation of perivascular macrophages
Abstract
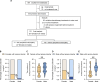
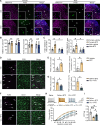
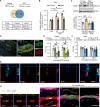
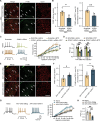
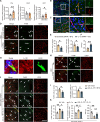
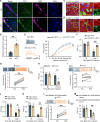
Sex differences in the pathogenesis of a variety of diseases have drawn increasing attention. However, it remains unclear whether such differences exist in chemotherapy-induced neuropathic pain. Here, we conducted a retrospective analysis of clinical case data and found that peripheral sensory disorders occurred earlier in females than in males following bortezomib (BTZ) treatment in patients with multiple myeloma. BTZ treatment led to an early elevation of intercellular adhesion molecule-1, which triggered the infiltration of peripheral monocytes into the perivascular region of the spinal cord in female mice. The CC-chemokine ligand 1 released by infiltrating macrophages directly activated neurons or indirectly activated neurons by enhancing the astrocyte activity, ultimately leading to the earlier onset of BTZ-induced neuropathic pain in females. Together, clarifying the mechanism underlying the earlier onset of BTZ-induced neuropathic pain will contribute to the precise treatment of multiple myeloma in females.
Figures



References
-
- Beijers A. J., Oerlemans S., Mols F., Eurelings M., Minnema M. C., Vreugdenhil A., van de Poll-Franse L. V., The magnitude of neurotoxicity in patients with multiple myeloma and the impact of dose modifications: Results from the population-based PROFILES registry. Ann. Hematol. 96, 653–663 (2017). - PubMed
-
- Cowan A. J., Green D. J., Kwok M., Lee S., Coffey D. G., Holmberg L. A., Tuazon S., Gopal A. K., Libby E. N., Diagnosis and management of multiple myeloma: A review. JAMA 327, 464–477 (2022). - PubMed
-
- Midavaine É., Côté J., Marchand S., Sarret P., Glial and neuroimmune cell choreography in sexually dimorphic pain signaling. Neurosci. Biobehav. Rev. 125, 168–192 (2021). - PubMed
-
- Mogil J. S., Sex differences in pain and pain inhibition: Multiple explanations of a controversial phenomenon. Nat. Rev. Neurosci. 13, 859–866 (2012). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

