Aggregation of α-synuclein splice isoforms through a phase separation pathway
- PMID: 40238878
- PMCID: PMC12002138
- DOI: 10.1126/sciadv.adq5396
Aggregation of α-synuclein splice isoforms through a phase separation pathway
Abstract
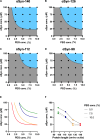
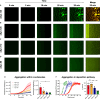
The aggregation of α-synuclein (αSyn) is associated with Parkinson's disease and other related synucleinopathies. Considerable efforts have thus been directed at understanding this process. However, the recently discovered condensation pathway, which involves the formation of phase-separated liquid intermediate states, has added further complexity. In parallel, it has been reported that different αSyn splice isoforms may be implicated in aggregate formation in disease. In this study, we compare the phase behavior of four αSyn isoforms (αSyn-140, αSyn-126, αSyn-112, and αSyn-98). Using different biophysical tools including confocal microscopy, kinetic assays and microfluidic-based approaches, we find stark differences between the four systems in their propensities to undergo phase separation and aggregation. Furthermore, we show that even small amounts of αSyn-112, one of the predominant isoforms after αSyn-140, can affect the phase separation of αSyn-140. These results highlight the importance of conducting further investigations to elucidate the role of alternative splicing in synucleinopathies.
Figures




References
-
- Spillantini M. G., Schmidt M. L., Lee V. M.-Y., Trojanowski J. Q., Jakes R., Goedert M., α-Synuclein in Lewy bodies. Nature 388, 839–840 (1997). - PubMed
-
- Poewe W., Seppi K., Tanner C. M., Halliday G. M., Brundin P., Volkmann J., Schrag A.-E., Lang A. E., Parkinson disease. Nat. Rev. Dis. Primers 3, 1–21 (2017). - PubMed
-
- Balestrino R., Schapira A., Parkinson disease. Eur. J. Neurol. 27, 27–42 (2020). - PubMed
-
- Aarsland D., Batzu L., Halliday G. M., Geurtsen G. J., Ballard C., Ray Chaudhuri K., Weintraub D., Parkinson disease-associated cognitive impairment. Nat. Rev. Dis. Primers. 7, 1–21 (2021). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

