A direct effect of the hematocrit on blood glucose: Evidence from hypoxia- and erythropoietin-treated mice
- PMID: 40238885
- PMCID: PMC12002128
- DOI: 10.1126/sciadv.adt7366
A direct effect of the hematocrit on blood glucose: Evidence from hypoxia- and erythropoietin-treated mice
Abstract
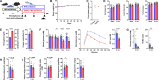
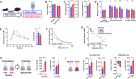
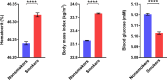
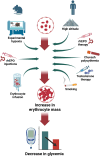
Blood glucose is lower in mountain dwellers living under low partial oxygen pressure. We show that obese mice maintained under hypoxia exhibit a delayed but distinct decrease in blood glucose with improved insulin sensitivity, which is independent of changes in body weight. This effect of hypoxia is mediated by erythropoiesis and is a direct result of the rising hematocrit, which could be due to erythrocytes acting as carriers of glucose units in the blood. Glucose lowering by the red cell mass is evidenced by a prompt decrease in glycemia in mice receiving a blood transfusion. Furthermore, life under hypoxia as well as treatment with erythropoietin reduce glycemia also in mice expressing the erythropoietin receptor exclusively in hematopoietic cells, which contrasts with previous assumptions attributing metabolic actions of erythropoietin to direct action on nonhematopoietic tissues. Our results provide a rationale for associations between hematocrit and blood glucose in humans under anti-anemic therapy, polycythemia, smoking, and high-altitude exposure.
Figures



References
-
- Lindgarde F., Ercilla M. B., Correa L. R., Ahren B., Body adiposity, insulin, and leptin in subgroups of Peruvian Amerindians. High Alt. Med. Biol. 5, 27–31 (2004). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

