Placental Endocannabinoid System: Focus on Preeclampsia and Cannabis Use
- PMID: 40238905
- PMCID: PMC12002044
- DOI: 10.1161/HYPERTENSIONAHA.125.24934
Placental Endocannabinoid System: Focus on Preeclampsia and Cannabis Use
Abstract
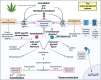
Background: The endocannabinoid system (ECS) plays an important role in the early stages of pregnancy, while cannabis use during pregnancy associates with a greater risk of preeclampsia. This study quantified the placental ECS component mRNA levels in gestational age-matched healthy pregnant women, women with preeclampsia, and women who used cannabis throughout their pregnancy. Next, it compared the effects of the endogenous ECS agonists anandamide and 2-arachidonoylglycerol with those of the cannabinoid receptor type 1 and 2 agonists HU-210 and HU-308 in chorionic plate arteries.
Methods: Placental mRNA levels were quantified by quantitative polymerase chain reaction. Vascular reactivity was studied with and without selective cannabinoid receptor type 1 and 2 antagonists.
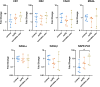
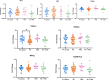
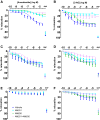
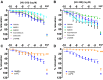
Results: mRNA levels of 1,2-diacylglycerol lipase α, responsible for 2-arachidonoylglycerol generation, were lowered in preeclampsia, while mRNA levels of the anandamide-synthesizing enzyme N-acyl phosphatidylethanolamine-specific phospholipase D were upregulated in cannabis users. Anandamide-induced relaxation in healthy pregnancy was mediated via cannabinoid receptors type 1 and 2, while 2-arachidonoylglycerol induced relaxation via cannabinoid receptor type 1. In preeclampsia, the effects of anandamide and 2-arachidonoylglycerol were unaltered but no longer involved cannabinoid receptors, while in cannabis users their effects were absent. HU-210 and HU-308 relaxed healthy, but not preeclamptic vessels. The NO donor sodium nitroprusside similarly relaxed healthy and preeclamptic vessels, while its effects in cannabis users were greatly reduced.
Conclusions: The ECS is disturbed in preeclampsia, and endogenous ECS agonists lose their capacity to dilate in cannabis users, while such use also diminishes NO signaling. These data provide mechanistic evidence against cannabis use during pregnancy.
Keywords: anandamide; cannabis; endocannabinoids; placenta; pre-eclampsia.
Conflict of interest statement
None.
Figures
Similar articles
-
Effects of cannabis tetrahydrocannabinol on endocannabinoid homeostasis in human placenta.Arch Toxicol. 2019 Mar;93(3):649-658. doi: 10.1007/s00204-019-02389-7. Epub 2019 Jan 18. Arch Toxicol. 2019. PMID: 30659320
-
The endocannabinoids anandamide and 2-arachidonoylglycerol modulate the expression of angiogenic factors on HTR8/SVneo placental cells.Prostaglandins Leukot Essent Fatty Acids. 2022 May;180:102440. doi: 10.1016/j.plefa.2022.102440. Epub 2022 Apr 21. Prostaglandins Leukot Essent Fatty Acids. 2022. PMID: 35490598
-
The fundamental role of the endocannabinoid system in endometrium and placenta: implications in pathophysiological aspects of uterine and pregnancy disorders.Hum Reprod Update. 2020 Jun 18;26(4):586-602. doi: 10.1093/humupd/dmaa005. Hum Reprod Update. 2020. PMID: 32347309 Free PMC article. Review.
-
Activation of CB1 receptors by 2-arachidonoylglycerol attenuates vasoconstriction induced by U46619 and angiotensin II in human and rat pulmonary arteries.Am J Physiol Regul Integr Comp Physiol. 2017 Jun 1;312(6):R883-R893. doi: 10.1152/ajpregu.00324.2016. Epub 2017 Mar 29. Am J Physiol Regul Integr Comp Physiol. 2017. PMID: 28356298
-
Why do cannabinoid receptors have more than one endogenous ligand?Philos Trans R Soc Lond B Biol Sci. 2012 Dec 5;367(1607):3216-28. doi: 10.1098/rstb.2011.0382. Philos Trans R Soc Lond B Biol Sci. 2012. PMID: 23108541 Free PMC article. Review.
References
-
- Fantauzzi MF, Aguiar JA, Tremblay BJM, Mansfield MJ, Yanagihara T, Chandiramohan A, Revill S, Ryu MH, Carlsten C, Ask K, et al. . Expression of endocannabinoid system components in human airway epithelial cells: impact of sex and chronic respiratory disease status. ERJ Open Res. 2020;6:00128–02020. doi: 10.1183/23120541.00128-2020 - PMC - PubMed
-
- Marzo VD. Anandamide serves two masters in the brain. Nat Neurosci. 2010;13:1446–1448. doi: 10.1038/nn1210-1446 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

