PRERISK Study: A Randomized Controlled Trial Evaluating a sFlt-1/PlGF-Based Calculator for Preeclampsia Hospitalization
- PMID: 40238908
- PMCID: PMC12002045
- DOI: 10.1161/HYPERTENSIONAHA.124.24386
PRERISK Study: A Randomized Controlled Trial Evaluating a sFlt-1/PlGF-Based Calculator for Preeclampsia Hospitalization
Abstract
Background: A model based on the soluble Fms-like tyrosine kinase-1/placental growth factor ratio, gestational age, and the urinary protein-to-creatinine ratio (PRERISK calculator) has been developed to predict preeclampsia-related maternal-fetal complications. Here, we tested whether this model can reduce hospital admissions without increasing complication rates among women with suspected or confirmed preeclampsia.
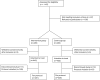
Methods: In this multicenter, open-label, randomized controlled trial conducted at 5 Dutch medical centers, women with suspected or confirmed preeclampsia were randomly assigned to the intervention group, where admission was guided by the PRERISK score using a 5% cutoff, or to the control group receiving routine care with a concealed PRERISK score. Two co-primary outcomes were the incidence of maternal-fetal preeclampsia-related complications (noninferiority) and the proportion of women with a hospitalization ratio (=admission days/inclusion days) ≤0.05 (superiority).
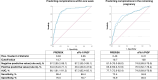
Results: The intervention and control groups included 442 and 435 women, respectively. In the intention-to-treat analysis, complications occurred in 41.6% of the intervention group versus 39.5% of the control group (adjusted relative risk 1.06 [95% CI, 0.92-1.22]; P=0.43). The proportion of women achieving a hospitalization ratio ≤0.05 was 23.6% in the intervention group and 26.3% in the control group (adjusted relative risk, 0.89 [95% CI, 0.71-1.13]; P=0.34). The latter was comparable in the per-protocol analysis (adjusted relative risk, 0.87 [95% CI, 0.64-1.19]; P=0.38), while in this analysis, complications occurred in 47.8% of the intervention group (n=251) versus 41.7% of the control group (n=365; adjusted relative risk 1.19 [95% CI, 1.03-1.38]; P=0.02).
Conclusions: Routine screening with the PRERISK score and a 5% cutoff in patients with suspected or confirmed preeclampsia does not decrease hospitalization and is therefore not recommended.
Registration: URL: https://onderzoekmetmensen.nl/nl/trial/48687; Unique identifier: NL63386.078.17, NL-OMON48687.
Keywords: placental growth factor; pre-eclampsia; pregnancy; risk assessment; soluble fms-like tyrosine kinase-1.
Conflict of interest statement
None.
Figures
References
-
- Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res. 2019;124:1094–1112. doi: 10.1161/CIRCRESAHA.118.313276 - PubMed
-
- Hypertension in pregnancy. Report of the American College of obstetricians and gynecologists’ task force on hypertension in pregnancy. Obstet Gynecol. 2013;122:1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88 - PubMed
-
- Magee LA, Brown MA, Hall DR, Gupte S, Hennessy A, Karumanchi SA, Kenny LC, McCarthy F, Myers J, Poon LC, et al. . The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis and management recommendations for international practice. Pregn Hypertens. 2022;27:148–169. doi: 10.1016/j.preghy.2021.09.008 - PubMed
-
- Zhang J, Klebanoff MA, Roberts JM. Prediction of adverse outcomes by common definitions of hypertension in pregnancy. Obstet Gynecol. 2001;97:261–267. doi: 10.1016/s0029-7844(00)01125-x - PubMed
-
- von Dadelszen P, Payne B, Li J, Ansermino JM, Broughton Pipkin F, Cote AM, Douglas MJ, Gruslin A, Hutcheon JA, Joseph KS, et al. ; PIERS Study Group. Prediction of adverse maternal outcomes in pre-eclampsia: development and validation of the fullPIERS model. Lancet. 2011;377:219–227. doi: 10.1016/S0140-6736(10)61351-7 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous

