Intronic FGF14 GAA repeat expansions impact progression and survival in multiple system atrophy
- PMID: 40239008
- PMCID: PMC12404725
- DOI: 10.1093/brain/awaf134
Intronic FGF14 GAA repeat expansions impact progression and survival in multiple system atrophy
Abstract
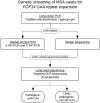
Partial phenotypic overlap has been suggested between multiple system atrophy and spinocerebellar ataxia 27B, the autosomal dominant ataxia caused by an intronic GAA•TTC repeat expansion in FGF14. In this study, we investigated the frequency of FGF14 GAA•TTC repeat expansion in clinically diagnosed and pathologically confirmed multiple system atrophy cases. We screened 657 multiple system atrophy cases (193 clinically diagnosed and 464 pathologically confirmed) and 1003 controls. The FGF14 repeat locus was genotyped using long-range PCR and bidirectional repeat-primed PCRs, and expansions were confirmed with targeted long-read Oxford Nanopore Technologies sequencing. We identified 19 multiple system atrophy cases carrying an FGF14 GAA≥250 expansion (2.89%, n = 19/657), a significantly higher frequency than in controls (1.40%, n = 12/1003) (P = 0.04). Long-read Oxford Nanopore Technologies sequencing confirmed repeat sizes and polymorphisms detected by PCR, with high concordance (Pearson's r = 0.99, P < 0.0001). Seven multiple system atrophy patients had a pathogenic FGF14 GAA≥300 expansion (five pathologically confirmed and two clinically diagnosed), and 12 had intermediate GAA250-299 expansion (six pathologically confirmed and six clinically diagnosed). A similar proportion of cerebellar-predominant and parkinsonism-predominant multiple system atrophy cases had FGF14 expansions. Multiple system atrophy patients carrying an FGF14 GAA≥250 expansion exhibited severe gait ataxia, autonomic dysfunction and parkinsonism, in keeping with a multiple system atrophy phenotype, with a faster progression to falls (P = 0.03) and regular wheelchair use (P = 0.02) in comparison to the multiple system atrophy cases without FGF14 GAA expansion. The length of the GAA•TTC repeat expansion lengths was inversely correlated with survival in multiple system atrophy patients (r = -0.67; P = 0.02) but not with age of onset. Therefore, screening for FGF14 GAA•TTC repeat expansion should be considered for multiple system atrophy patients with rapid loss of mobility and for complete diagnostic accuracy at inclusion in disease-modifying multiple system atrophy drug trials.
Keywords: FGF14 GAA ataxia; multiple system atrophy; spinocerebellar ataxia 27B.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
J.B.R. has undertaken remunerated consultancy or advisory board roles for Astronautx, Astex, Asceneuron, Alector, CumulusNeuro, Curasen, Eisai, Prevail and SV Health and has received academic grant income from AstraZeneca, Lilly, GSJ and Janssen as partners in Dementias Platform UK. M.S. has received consultancy honoraria from Ionis, UCB, Prevail, Orphazyme, Biogen, Servier, Reata, GenOrph, AviadoBio, Biohaven, Zevra, Lilly and Solaxa, all unrelated to the present manuscript. W.G.M. has received consultancy fees from Lundbeck, Takeda, Inhibikase, GE and Koneksa. H.R.M. is employed by UCL; in the last 12 months he reports paid consultancy from Roche, Aprinoia, AI Therapeutics and Amylyx; lecture fees/honoraria from BMJ, Kyowa Kirin and the Movement Disorders Society; and research grants from Parkinson's UK, Cure Parkinson's Trust, PSP Association, Medical Research Council and Michael J. Fox Foundation. Dr Morris is a co-applicant on a patent application related to C9ORF72-Method for diagnosing a neurodegenerative disease (PCT/GB2012/052140). T.F. has served on Advisory Boards for Peptron, Voyager Therapeutics, Handl therapeutics, Gain therapeutics, Living Cell Technologies, Abbvie, Bluerock, Bayer and Bial. He has received honoraria for talks sponsored by Bayer, Bial, Profile Pharma, Boston Scientific and Novo Nordisk. All other authors report no competing interests.
Figures



References
-
- Papp MI, Kahn JE, Lantos PL. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome). J Neurol Sci. 1989;94:79–100. - PubMed
-
- Spillantini MG, Anthony Crowther R, Jakes R, et al. Filamentous α-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci Lett. 1998;251:205–208. - PubMed
MeSH terms
Substances
Grants and funding
- ABN 540868/Association of British Neurologists' Academic Clinical Training Research Fellowship
- 565908/Association of British Neurologists' Academic Clinical Training Research Fellowship
- University College London Hospitals Biomedical Research Centre
- Multiple System Atrophy Coalition
- European Academy of Neurology
LinkOut - more resources
Full Text Sources
Miscellaneous

